Abstract
Loss of interleukin (IL)-7 or the IL-7 receptor alpha (IL-7Rα, CD127) results in severe immunodeficiencies in mice and humans. To more precisely identify signals governing IL-7 function in vivo, we have disrupted the IL-7Rα Y449XXM motif in mice by knock-in mutagenesis (IL-7Rα449F). Thymic precursors were reduced in number in IL-7Rα449F mice, but in marked contrast to IL-7Rα−/− knockout mice, thymocytes and peripheral T cells developed normally. Strikingly, Listeria infection revealed that CD4 and CD8 T cells had different requirements for IL-7Rα signals. CD4 T cells failed to mount a primary response, but despite normal CD8 primary responses, maintenance of CD8 memory was impaired in IL-7Rα449F mice. Furthermore, we show that Bcl-2 is IL-7Rα Y449 independent and insufficient for IL-7–mediated maintenance of CD8 memory.
The cytokine IL-7 is essential for development of murine pro–B and pro–T cells (1, 2) and normal function and survival of peripheral T cells (3). Loss of IL-7 or either component of its heterodimeric receptor, IL-7Rα (CD127) or γ common chain (γc; CD132), results in severe combined immunodeficiency in mice (1, 2) and T cell immunodeficiency in humans (4). Furthermore, IL-7 and its signaling have been shown to be essential in homeostasis and function of peripheral T cells (3).
Upon pathogen exposure, antigen-specific T cells undergo clonal expansion and differentiate into effector cells to clear the infection. To maintain homeostasis within the peripheral T cell niche, the majority of these effector cells die by apoptosis. However, 5–10% of pathogen-specific clones are retained long term as memory T cells to mount a more efficient response upon subsequent exposure. Memory T cells are believed to last up to 15 yr in humans as seen in smallpox immunization (5), but the mechanism by which this occurs is an open question. Evidence suggests that signals from the γc cytokines IL-7 and IL-15 are critical in the maintenance of memory CD8 T cells: IL-15 providing signals to drive basal levels of proliferation (6–8) and IL-7 providing survival signals by maintaining expression of Bcl-2 (9).
In situations of lymphopenia, such as after chemotherapy and in cases of viral infection, T cells respond by initiating a proliferative program to replenish host lymphoid cellularity (10–12). IL-7 is up-regulated in this situation, suggesting that increased availability of IL-7 plays a major role in altered homeostatic conditions (13). IL-7 signaling has been shown to have a role in homeostatic proliferation (HP) of both naive and memory T cells (9, 12, 14–16). Naive CD4 and CD8 T cells rely on both IL-7 and TCR signaling for HP (9, 17, 18). In contrast, HP of memory phenotype CD8+CD44hi T cells is primarily cytokine driven, using IL-15 to drive HP, although supraphysiological doses of IL-7 have been shown to be sufficient in the absence of IL-15 (15, 19, 20). The requirements for memory CD4 T cell HP are less clear and may require a synergy of both IL-7 and TCR signaling for efficient responses (14). Studies have shown that naive T cells develop a memory-like phenotype during HP and have features of functional memory T cells (21), raising the question of whether molecular pathways involved in HP are similar to those that generate classical memory T cells after infection.
The γc is constitutively expressed at low levels on lymphocytes, but IL-7Rα expression is restricted to specific stages of differentiation to mediate cellular responses (22–24). Within the T cell lineage, IL-7Rα is expressed in the early stages of development on CD4−CD8− double negative (DN) cells, consistent with IL-7 cytokine or receptor deficiencies resulting in a thymic developmental block at these early stages and a paucity of thymocytes differentiating into CD4+CD8+ double positive (DP), CD4, or CD8 single positive (SP) or peripheral T cells (1, 2). IL-7Rα expression is down-regulated on CD4+CD8+ DP thymocytes, likely to prevent transduction of survival signals to clones that do not meet the requirements of positive selection (25). IL-7Rα expression is then resumed and maintained on surviving CD4+ and CD8+ SP thymocytes (24) and is involved in survival and functional responses of mature T cells (3, 9). It is thought that the primary function of IL-7 signaling in the DN stages is to provide survival signals because ectopic expression of the antiapoptotic Bcl-2 protein in IL-7Rα−/− mice has been shown to rescue T cell development (26, 27). Indeed, Bcl-2 expression closely correlates with IL-7Rα expression during thymic maturation.
IL-7Rα activation reportedly initiates at least two separate signaling cascades, the Jak/STAT pathway and the phospatidylinositol-3 (PI3) kinase/Akt pathway. Phosphorylation of tyrosine (Y) residues of the IL-7Rα and γc by their constitutively associated Jak kinases leads to recruitment of Src homology 2 domain effectors such as STAT5 and PI3 kinase (24). Each of these cascades is thought to separately regulate Bcl-2 family members to promote survival of activated cells and increase resistance to apoptosis (28, 29). The distal three of four IL-7Rα cytoplasmic tyrosines (Y390, 401, 449, and 456) are conserved between mice and humans. Of these, Y449, nested in a YXXM motif, is necessary for STAT5 activation and regulation of PI3 kinase in B cells and human thymocytes and T cells (30–32). Thus, Y449 is a critical residue in initiation of signaling cascades leading to cell differentiation, proliferation, and survival (24, 29, 31). Y401 is nested in a conserved YXXL motif that is less widely distributed and is nonessential for thymocyte development and STAT5 activation (29). Y456 is in the least optimal setting because it is not in an acidic context and is less than four amino acids from the carboxyl terminus of the receptor.
The severe developmental defect in IL-7−/− and IL-7Rα−/− mice hampers further analysis of the role of this essential cytokine in T cell function. To this end, we have generated an IL-7Rα “knock-in” mouse in which the gene has a site-specific mutation of Y449 to phenylalanine (F) (IL-7Rα449F) to specifically abrogate Y449XXM-derived signals. Like IL-7Rα−/− mice, lymphocyte development is disrupted at early stages but is substantially restored in IL-7Rα449F mice revealing a significant role for IL-7Rα Y449–independent events. This IL-7Rα Y449–independent increase in lymphocyte cellularity is recapitulated in the periphery, and IL-7Rα449F mice develop significantly larger pools of peripheral T cells than IL-7Rα−/− mice. Thus, this model enables investigation of the role of IL-7Rα signaling in an in vivo context. In addition, known signaling pathways can be separated into Y449 dependence and independence and functional outcomes described in these terms. Analysis of IL-7Rα449F T cells revealed a critical role for Y449-mediated signaling in physiologically relevant processes such as HP, generation of a primary CD4 T cell immune response, and maintenance of antigen-specific CD8 memory T cells. Remarkably, Bcl-2 regulation is near normal in IL-7Rα449F mice, and we conclude that maintenance of Bcl-2 expression is not sufficient for survival of CD8 memory T cells as has been previously suggested (9). Our analysis provides genetic separation of IL-7–mediated CD8 memory cell maintenance from survival provided by Bcl-2.
RESULTS
Generation of IL-7Rα449F mice
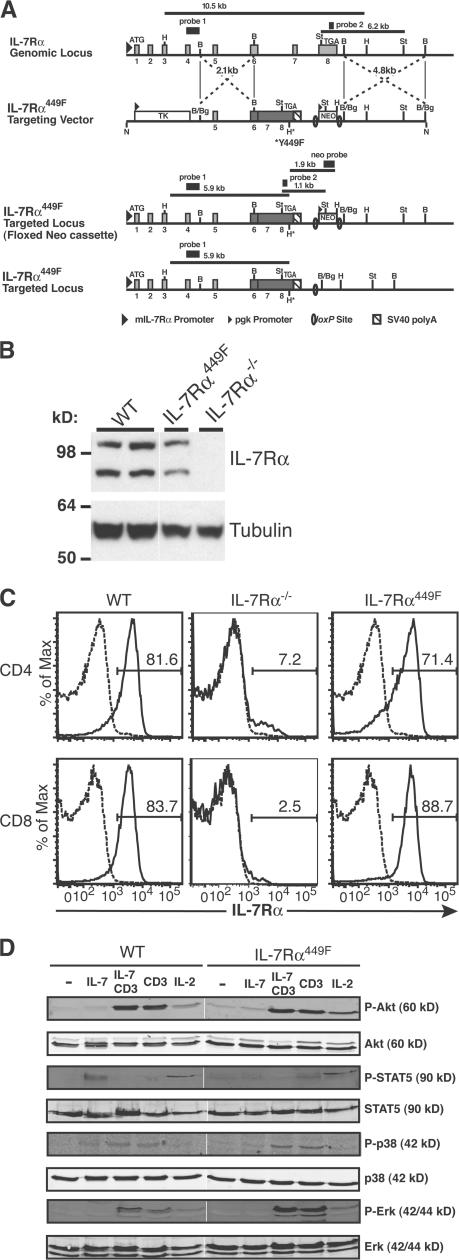
To analyze the role of signaling effectors downstream of IL-7Rα on in vivo T lymphocyte function, we used homologous recombination with a replacement vector to generate the IL-7Rα449F knock-in mice (Fig. 1 A; and Fig. S1 and Supplemental Materials and methods available at http://www.jem.org/cgi/content/org/jem.20061871/DC1). The targeted IL-7Rα locus retained the endogenous promoter and upstream exons; however, exons 6–8 were replaced with cDNA encoding the transmembrane and cytoplasmic domains, with disruption of the YXXM motif by the Y449F substitution (Fig. 1 A). The phosphorylated IL-7Rα YXXM motif has been shown to be the binding site for the Src homology 2 domain of PI3 kinase and activation of downstream pathways (30–32). The NeoR selection marker was deleted by Cre-mediated recombination to eliminate potential promoter effects. Initial analysis of mutant IL-7Rα protein expression from thymocyte lysates indicated patterns comparable to WT thymocytes with mature polypeptides (90 kD) (Fig. 1 B). Most importantly, we determined that the Y449F knock-in mutation did not affect surface expression of IL-7Rα on peripheral CD4 and CD8 T cells (Fig. 1 C). Furthermore, signaling analysis of WT and IL-7Rα449F–cultured T cell blasts showed that TCR activation of the Erk and p38 MAPK pathways was unaffected, but that IL-7 activation of STAT5 requires IL-7Rα Y449 (Fig. 1 D). Interestingly, IL-7 by itself does not induce phosphorylation of Akt (Fig. 1 D) or S6 kinase (unpublished data) in primary murine T cells at the time points tested.
Figure 1.
Generation of IL-7Rα449F knock-in mouse. (A) A targeting vector carrying a partial cDNA of the IL-7Rα transmembrane and cytoplasmic domains with Y449F site-specific mutation (*) were used for homologous recombination in ES cells. WT, targeted, floxed and targeted (IL-7Rα449F) loci are shown (not to scale). Light gray boxes, exons; numerals below thick black lines, exon numbers; dark gray box, IL-7Rα cDNA. Sequences for neomycin (NEO) and thymidine kinase (TK) are shown. H, HindIII; B, BamHI; St, StuI; Bg, BglII; N, NotI, H*, HindIII site introduced. Probe 1 is a 0.65-kb EcoRI-BamHI fragment upstream of the short arm (Supplemental Materials and methods). Probe 2 is a 202-bp fragment amplified from exon 8, and the Neo probe is a 1.6-kb HindIII-SpeI fragment from the targeting vector. (B) Expression of IL-7Rα is maintained in IL-7Rα449F thymocytes. Thymocyte lysates were prepared from WT, IL-7Rα449F, and IL-7Rα−/− mice, and analyzed by immunoblot. Protein loading is indicated by the antitubulin immunoblot (bottom). (C) Site-specific mutation of IL-7Rα Y449 does not affect expression of IL-7Rα on peripheral CD4 and CD8 T cells as assessed by flow cytometry. Experiment is representative of three mice for WT, IL-7Rα−/−, and IL-7Rα449F in two independent experiments. Isotype antibody, dashed trace; anti–IL-7Rα, solid trace. (D) IL-7Rα Y449F mutation abrogates activation of STAT5, but identifies activation of the p38 MAPK pathway as Y449 independent. Neither Akt nor Erk are activated by IL-7 in T cells, but are efficiently activated by α-CD3 and IL-2 stimulation.
Development of early hematopoietic lineages in IL-7Rα449F mice
To perform a comprehensive analysis of hematopoietic development in IL-7Rα449F mice, we investigated the effect of loss of IL-7Rα Y449 signaling during development of early hematopoietic lineages using flow cytometry. We found the total number of bone marrow hematopoietic stem cells (HSCs) and derivatives were unaffected by the mutation (Fig. 2 A). Once again, we determined that the Y449F knock-in mutation does not affect surface expression of IL-7Rα on HSCs (Fig. 2 B). However, the earliest thymic T cell progenitors were severely impaired in both IL-7Rα mutants as indicated by a 20-fold decrease in the total number of early thymic progenitors (ETPs; characterized as Lin−CD3−CD4−CD8−CD25−CD117+ [33]) in IL-7Rα449F and IL-7Rα−/− mice in comparison to WT (Fig. 2 C).
Figure 2.
Bone marrow progenitor populations and IL-7Rα expression are normal in IL-7Rα449F mice. (A) Bone marrow progenitors develop at similar frequencies (left) and total cell numbers (right) in mice with targeted IL-7Rα449F mutation. Bar graphs are gated on Lin−Sca1+c-kit+ HSCs and their Lin−Sca1loc-kit+ derivatives (post-HSCs). (B) IL-7Rα is expressed normally on IL-7Rα449F bone marrow populations as determined by FACS analysis. Isotype antibody, dashed trace; anti–IL-7Rα, solid trace. (C) The frequency of ETPs is decreased in both IL-7Rα−/− and IL-7Rα449F thymi compared with WT. FACS plots shown are gated on Lin−CD44+CD25− DNI populations. WT, black bars; IL-7Rα−/−, unfilled bars; IL-7Rα449F, hatched bars. All experiments are representative of at least three mice for WT, IL-7Rα−/−, and IL-7Rα449F in two independent experiments.
IL-7Rα449F thymocytes bypass a developmental block
Complete disruption of IL-7Rα results in decreased survival of early T lymphocyte progenitors causing a severe cytopenia that precludes detailed analysis of its role in peripheral T cells (1, 2, 26). In contrast, IL-7Rα449F mice showed only a fourfold decrease in thymic cellularity compared with WT despite the severe ETP defect (Fig. 3 A, right), suggesting a role for IL-7Rα Y449-independent signaling in thymocyte development. In agreement with this hypothesis, the distribution of thymocyte subsets in IL-7Rα449F mice was indistinguishable from WT (Fig. 3 A, left). To further examine thymocyte development, WT, IL-7Rα449F, and IL-7Rα−/− thymocytes were analyzed by expression of CD44 and CD25 in the CD4−CD8− DN compartment using flow cytometry. At all DN stages (DNI, CD4−CD8−CD44+CD25−; DNII, CD4−CD8−CD44+CD25+; DNIII, CD4−CD8−CD44− CD25+; DNIV, CD4−CD8−CD44−CD25−), the defects in IL-7Rα449F were less severe than in IL-7Rα−/− mice (Fig. 3 B, left). In addition, IL-7Rα449F mice had significantly higher numbers of DP thymocytes than IL-7Rα−/− mice (Fig. 3 A). This could be explained by the observation that IL-7Rα449F and WT mice showed similar production of DP thymocytes from DNIV precursors,whereas IL-7Rα−/− DNIV cells encountered a severe developmental block at this stage (Fig. 3 B, right). This indicates an IL-7Rα Y449–independent compensatory mechanism that contributes to restoration of normal thymocyte development and partial rescue of total T cell numbers. The role of IL-7Rα signaling in positive selection was examined by determining the frequency of TCR-β+ DP thymocytes that had downregulated heat stable antigen (HSA) (Fig. 3 C) or upregulated the maturation marker CD69 (unpublished data). These analyses showed that neither complete deletion nor mutation of IL-7Rα resulted in impaired positive selection, and we conclude that IL-7Rα is nonessential in this process.
Figure 3.
An early thymocyte defect is bypassed in IL-7Rα449F mice. (A) FACS analysis shows that IL-7Rα449F thymocytes develop DN, DP, CD4 SP, and CD8 SP populations in frequencies similar to WT mice (left). Total cellularity in IL-7Rα449F mice is reduced in comparison to WT but significantly higher than in IL-7Rα−/− littermates (right). (B) DN thymocyte development is affected by IL-7Rα449F mutation (left). Mean cell numbers are shown in quadrants. The transition to DP thymocytes is unaffected in IL-7Rα449F mice (right). (C) Positive selection is IL-7Rα independent as shown by frequency of TCR-β+ HSAlo cells in WT, IL-7Rα−/−, and IL-7Rα449F DP thymocytes. (D) γδ T cell development is impaired in the absence of IL-7Rα Y449 signaling. Bar charts show the number of anti-γδ TCR+ lymphocytes in the thymus. (A, B, and D) WT, black bars; IL-7Rα−/−, unfilled bars; IL-7Rα449F, hatched bars. (E) BrdU uptake revealed cell cycling is increased in DNIII and DP stages but decreased in DNIV IL-7Rα449F thymocytes. Isotype antibody, dashed trace; anti-BrdU, solid trace. (F) Competitive repopulation shows IL-7Rα449F– derived cells were able to differentiate normally but are poorly competitive. Bar chart (left) and contour plots (right) are gated on CD90.1+ CD45.2+ (black bar) and CD90.1− CD45.2+ (hatched bar) thymocytes. Numbers in bar charts represent the mean thymocyte recovery from each genotype. All experiments are representative of at least three mice for WT and IL-7Rα449F. IL-7Rα−/− samples were pooled from three to six mice.
Commitment to the γδ T cell lineage is IL-7Rα dependent (34) and occurs late in the DNII to DNIII stage (35). We assessed γδ development by flow cytometry by staining total thymocytes with anti-γδ antibody and a lineage cocktail. Our data showed that γδ development is Y449 dependent because their numbers were markedly diminished (Fig. 3 D). Collectively, our data shows that IL-7Rα Y449 signals play critical roles in both αβ and γδ T cell development.
To examine the basis of restored cellularity in IL-7Rα449F mice, cell turnover was determined by BrdU incorporation to monitor uptake by rapidly cycling DN thymocytes. We found IL-7Rα449F DNIV cells that lack surface expression of the mutated IL-7Rα (unpublished data) have markedly decreased BrdU uptake, whereas the DNIII and DP stages undergo more rapid cycling compared with WT (Fig. 3 E). Collectively, the data suggest that IL-7Rα–driven cell division in DN and DP thymocytes is Y449 independent.
To examine the severity of the IL-7Rα449F mutation on developmental potential, competitive repopulation experiments were performed. This analysis revealed that IL-7Rα449F thymocytes were less robust than their WT counterparts. Similar to what was seen in whole mice, when either WT or IL-7Rα449F whole bone marrow were delivered separately, IL-7Rα449F recipient thymic development resulted in fourfold lower cellularity than WT recipients (unpublished data). However, when WT (CD90.1+) and IL-7Rα449F (CD90.2+) whole bone marrow were delivered at a 1:1 ratio, the thymic output was comprised of <5% IL-7Rα449F–derived cells, although subset distribution was still normal (Fig. 3 F). Collectively, our data shows that knock-in disruption of IL-7Rα Y449 signals causes stage-specific defects, particularly dramatic at the ETP stage, but that these are bypassed to generate normal thymocyte subsets.
IL-7Rα Y449-independent signals are involved in peripheral T cell development
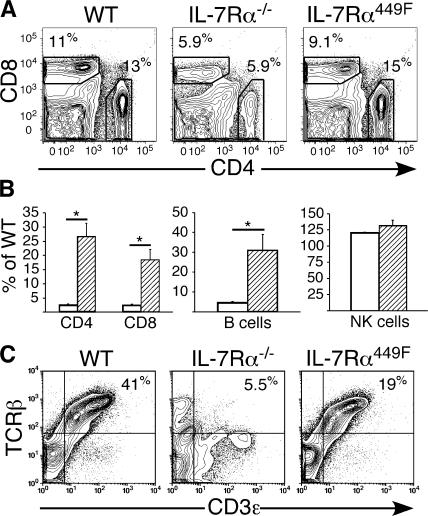
The severe developmental defect in IL-7Rα−/− thymocytes (1) causes a paucity of T cells in the periphery that confounds further analysis of the role of IL-7 in T cell function (3). When peripheral T cells were examined in IL-7Rα449F mice, we found that the relative subset distribution is similar between IL-7Rα449F and WT mice, whereas IL-7Rα−/− mice had a severe disruption of CD4 and CD8 T cell development (Fig. 4 A). Quantification of peripheral lymphocytes revealed that IL-7Rα449F mice had significantly lower total cell numbers than WT mice (CD4 T cells: 2.5 ± 0.3; CD8 T cells: 3.8 ± 0.6; B cells: 3.7± 1.0 fold decrease, n = 3) but that these subsets were higher than in IL-7Rα−/− mice, thus indicating that lymphocyte development is partially IL-7Rα Y449 independent (Fig. 4 B). Consistent with the expectation that this targeted mutation only affects IL-7Rα–mediated events, development of NK cells (NK1.1+CD3−) was unaffected in the spleens of both IL-7Rα449F and IL-7Rα−/− mice (Fig. 4 B). TCR-β and CD3ɛ expression levels on WT, IL-7Rα449F, and IL-7Rα−/− splenic T cells were analyzed by flow cytometry and found to be comparable between WT and IL-7Rα449F mice, whereas those from IL-7Rα−/− mice were severely deficient (Fig. 4 C). This shows that IL-7Rα Y449 signaling is dispensable for peripheral T cell maturation as assessed by antigen receptor expression.
Figure 4.
Disruption of IL-7Rα Y449 signaling only partially perturbs peripheral lymphocyte development. (A) Peripheral CD4 and CD8 T cells develop in IL-7Rα449F mice and confirm involvement of IL-7Rα Y449–independent pathways. (B) Quantification of FACS data shows that peripheral T and B cell development is reduced in IL-7Rα449F compared with WT but significantly higher than in IL-7Rα−/− littermates and supports a role for IL-7Rα Y449–independent signaling requirements. Natural killer cells (CD3−NK1.1+) are unaffected by abrogation of IL-7Rα Y449 signals. Data is expressed as the percentage of WT cellularity, IL-7Rα−/− (white bars), and IL-7Rα449F (hatched bars). (C) IL-7Rα Y449–independent signaling allows for accumulation of mature CD3+TCRβ+ T cells in the periphery. See Fig. S2.
To eliminate the possibility of altered expression of other γc receptor family members in IL-7Rα449F mice accounting for differences in peripheral T cell function, surface expression levels of cytokine receptor chains γc and IL-2/15Rβ were examined. Our data showed only marginal differences in IL-7Rα and γc expression between WT and IL-7Rα449F CD4 and CD8 T cells, although the frequency of IL-2/15Rβ–expressing cells was increased in both IL-7Rα449F and IL-7Rα−/− T cells (Fig. S2, available at http://www.jem.org/cgi/content/org/jem.20061871/DC1).
Homeostatic proliferation is IL-7Rα Y449 dependent
Because IL-7Rα449F mice are able to support T cell development, we sought to address what aspects of T cell function were impaired or retained. IL-7 has been shown to be involved in homeostatic proliferation of naive CD4 and CD8 T cells (9, 13, 14). To address the role of IL-7Rα Y449 in homeostatic proliferation, IL-7Rα449F and WT (CD45.2+) T cells were labeled with the mitotic tracker CFSE and transferred into either normal or irradiated recipient host mice (CD45.1+). In stark contrast to WT T cells, IL-7Rα449F T cells were unable to proliferate when transferred into acutely lymphopenic hosts as measured by CFSE dilution (Fig. 5). Indeed the deficiency was as severe as that in IL-7Rα−/− cells. A small pool of CD8 T cells undergo limited rounds of division, but these appear IL-7Rα independent because it was also observed with IL-7Rα−/− cells (Fig. 5). Our data strongly argue that homeostatic proliferation of both CD4 and CD8 T cells is dependent on signals initiated by IL-7Rα Y449.
Figure 5.
Signals from IL-7Rα Y449 are essential for IL-7–driven homeostatic proliferation. Experiments are representative of two to four recipient mice from WT, IL-7Rα449F, and IL-7Rα−/− T cells in two independent experiments. Irradiated hosts, black, filled trace; nonirradiated hosts, white trace.
IL-7Rα Y449 is essential for CD4 primary immune responses
To determine the role of IL-7Rα Y449 in generating a functional polyclonal T cell immune response in vivo, WT and IL-7Rα449F mice were infected with a recombinant Listeria monocytogenes strain expressing an immunodominant foreign, MHC class I (Kb)–restricted peptide, SIYRYYGL (rLM-SIY) (Priatel, J., L. Zenewicz, H. Shen, and H. Teh, personal communication). Primary responses to rLM-SIY were monitored at day 7, and antigen-specific T cells were enumerated by IFN-γ production in CD4 or CD8 T cells after in vitro stimulation with immunodominant MHC class II (listeriolysin O [LLO]190-201)– and MHC class I (SIY)–restricted peptides, respectively. Surprisingly, IL-7Rα449F CD4 T cells failed to generate a detectable primary immune response after infection (Fig. 6 A). In contrast, IL-7Rα449F CD8 T cells mounted a robust response that was equivalent to WT controls in total cell numbers (Fig. 6 A), independent of lower initial T cell numbers in IL-7Rα449F mice. In vitro anti-CD3/anti-CD28 TCR stimulation assays showed that IL-7Rα449F CD4 and CD8 T cells had impaired proliferation response to low, but not high, levels of TCR stimulation (Fig. 6 B) and this may account for the defective CD4 primary response.
Figure 6.
IL-7Rα Y449 is essential for the CD4 primary response to L. monocytogenes. (A) CD4 but not CD8 T cells require IL-7Rα Y449 for differentiation into effector cells. Bar charts represent total cell numbers of CD4+ IFN-γ+ or CD8+ IFN-γ+ T cells from WT (black bars) or IL-7Rα449F (hatched bars) spleens at day 7 after infection. (B) Decreased TCR proliferation in IL-7Rα449F CD4 and CD8 T cells at low concentrations of agonist stimulation. The doses of plate-bound α-CD3 are indicated. Histograms show representative results of one to three replicates. WT, solid trace; IL-7Rα449F, dashed trace. (C) Adoptively transferred WT Thy1.1 (CD90.1+) CD4 and CD8 T cells respond to rLM-SIY infection equally well in WT (CD90.1−) and IL-7Rα449F (CD90.1−) hosts. Bar charts represent the number of Thy1.1 antigen–specific T cells recovered from WT hosts (black bars) and IL-7Rα449F hosts (hatched bars). Data are representative of at least three infected mice of each genotype for each experiment.
To determine whether or not the decrease in B cells or an altered splenic compartment could account for the lack of CD4 T cell primary response in IL-7Rα449F mice, equal numbers of WT Thy1.1 (CD90.1+) T cells were adoptively transferred into WT and IL-7Rα449F CD90.2+ hosts. The mice were infected with rLM-SIY and responses measured at day 7 after infection by determining IFN-γ expression upon in vitro stimulation with the indicated peptides. The fact that WT CD90.1+ CD4 T cells responded strongly in IL-7Rα449F hosts (Fig. 6 C) confirmed that the IL-7Rα449F CD4 defect was T cell intrinsic and not a result of inefficient antigen presentation. These data show that CD4, but not CD8 T cells, require IL-7Rα Y449–mediated signals to generate primary responses to rLM-SIY.
IL-7Rα Y449 is essential for maintenance of CD8 T cell memory
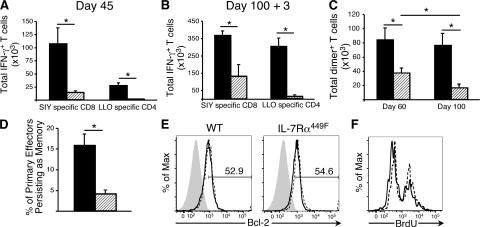
Previous reports have suggested that IL-7Rα is critical for the generation and/or maintenance of T cell memory. The fact that IL-7Rα449F mice mount a strong primary CD8 T cell response to the MHC class I–restricted SIY peptide enabled us to investigate whether the formation of CD8 T cell memory is dependent on IL-7Rα Y449. WT and IL-7Rα449F mice were infected with rLM-SIY and analyzed 45 d later for antigen-specific memory CD8 T cells by expression of IFN-γ in response to peptide. Despite generating equivalent numbers of CD8 T cell effectors as WT at day 7, IL-7Rα449F mice showed an eightfold reduction in memory CD8 T cell numbers compared with WT mice (Fig. 7 A). Not surprisingly, the severely impaired primary CD4 T cell response in IL-7Rα449F mice resulted in very few LLO-reactive memory CD4 T cells. This experiment indicates an essential role for IL-7Rα Y449 signaling in regulation of CD8 memory T cells.
Figure 7.
CD8 memory T cells require signals from IL-7Rα Y449 for long-term maintenance. (A) IL-7Rα449F CD8 T cells are defective in their ability to survive and generate a stable pool of antigen-specific memory cells. (B) Rechallenge with rLM-SIY at day 100 showed that recovery of antigen-specific CD8 memory T cells was significantly impaired in IL-7Rα449F mice. (C) Analysis of SIY-specific T cells at days 60 and 100 after rLM-SIY infection using SIY-loaded MHC–Ig dimer showed that IL-7Rα449F memory CD8 T cells numbers are impaired and decrease over time. Data shown is representative of four mice of each genotype. (D) Adoptive transfer of WT CD90.1+ CD4 and CD8 T cells allows generation of WT CD8 memory, but provision of WT CD4 T cell help is insufficient for IL-7Rα449F CD8 T cell memory. % of primary effectors persisting as memory = no. of d45 CD8+ Dimer+ / no. of d7 CD8+Dimer+ × 100. Data shown is based on four transplanted mice at each time point. (E) Intracellular Bcl-2 was measured in peptide-stimulated CD8+IFN-γ+ T cells of WT and IL-7Rα449F mice at memory stages. No significant difference was detected in frequency of Bcl-2+ cells. Isotype antibody, gray filled trace; unstimulated, dashed trace; SIY stimulated, solid trace. Data shown is representative of three mice of each genotype and two independent experiments. (F) BrdU incorporation is similar in WT and IL-7Rα449F memory CD8 T cells at day 45 after rLM-SIY infection. Histograms are gated on CD8+IFN-γ+ cells. Solid trace, WT; dashed trace, IL-7Rα449F. Data is representative of three WT and IL-7Rα449F mice.
To test the fitness of IL-7Rα449F memory T cells, WT and IL-7Rα449F mice previously immunized with rLM-SIY were homologously rechallenged at 100 d after infection. 3 d after reinfection, the IL-7Rα449F mice showed an increase in the number of SIY-specific CD8 T cells but failed to reach the same level as achieved by WT controls (Fig. 7 B).
Because IL-7Rα449F T cells have impaired proliferation at low dose stimulus of the TCR, the possibility existed that the number of IL-7Rα449F memory CD8 T cells was underestimated by assessing their ability to produce IFN-γ upon peptide challenge. To confirm our findings of decreased CD8 memory T cells, an H2-Kb MHC I–Ig fusion protein conjugated to PE (MHC–Ig dimer) was loaded with SIY peptide and used for detection of antigen-specific cells. As shown in Fig. 7 C, the number of IL-7Rα449F memory CD8 T cells remains significantly lower (2.5-fold) than WT at day 60, although the decrease is not quite as dramatic as that shown by IFN-γ induction. This suggests that antigen-specific IL-7Rα449F memory CD8 T cells have compromised TCR-mediated cytokine induction. Strikingly, when memory T cells were assayed at day 100 in a parallel experiment, the WT CD8 memory T cell numbers remained constant, whereas IL-7Rα449F CD8 memory T cell numbers continued to decrease (Fig. 7 C). These data demonstrate a requirement for IL-7Rα Y449 signals in maintenance and long term survival of memory CD8 T cells.
To address whether or not the lack of a primary CD4 T helper cell expansion in the IL-7Rα449F mice was affecting the generation of an efficient memory CD8 T cell compartment, we performed adoptive transfer experiments wherein WT CD90.1+ T cells were transferred into IL-7Rα449F (CD90.2+) hosts. This allows WT and IL-7Rα449F CD8 T cells to encounter antigen in the same environment and receive the same signals from activated WT CD4 T helper cells. WT and IL-7Rα449F antigen-specific CD8 T cells were enumerated using MHC–Ig dimer staining at day 7 and 45 after rLM-SIY infection. To account for differences in transplant versus host cell numbers within spleens, we normalized the number of antigen-specific CD8 memory T cells against the number of antigen-specific primary CD8 T cells and represented this as a percentage. The normal range of effector T cells that survive the contraction period to become memory cells is between 5 and 10%. We saw that >15% of WT but <5% of IL-7Rα449F effector CD8 T cells were maintained at memory stage at day 45 after infection (Fig. 7 D), refuting the hypothesis that lack of CD4 T cell help accounts for the defect in IL-7Rα449F CD8 T cell memory.
We therefore looked at possible cell intrinsic disruptions in IL-7Rα449F CD8 memory T cells. Memory T cells require a balance of cell survival and low-level proliferation for long-term maintenance. Previous studies have demonstrated a role for IL-7 in survival of memory CD8 T cells by regulating levels of the survival factor Bcl-2 (9, 36). Using intracellular staining and flow cytometry, we found that the levels of Bcl-2 in WT and IL-7Rα449F CD8+ IFN-γ+ memory T cells were equivalent after rechallenge (Fig. 7 E) and at day 45 after infection (not depicted), and therefore cannot account for the maintenance defect. IL-15 has been demonstrated to regulate proliferation of memory CD8 T cells (6–8), but it remained possible that the IL-7Rα449F memory CD8 T cell pool was decreasing over time because of decreased proliferation. In agreement with previous studies that cycling of memory cells is regulated by IL-15 (37), we found that the cycling of IL-7Rα449F memory CD8 T cells was not disrupted as shown by BrdU incorporation (Fig. 7 F). Thus, the defect in IL-7Rα449F memory CD8 T cells does not result from impaired cycling or from a lack of Bcl-2 survival signals.
STAT5, but not Bcl-2, is IL-7Rα Y449 dependent
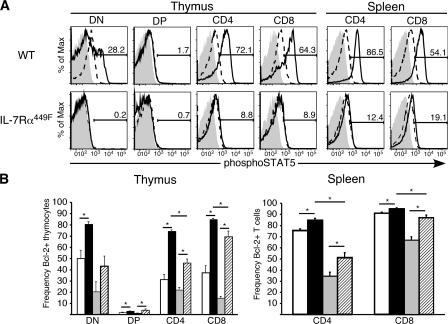
To more fully address the requirement of IL-7Rα Y449 in activation of known IL-7 signaling pathways, WT and IL-7Rα449F thymocytes and splenocytes were stimulated ex vivo with purified, recombinant IL-7 and analyzed by flow cytometry for phosphorylation of STAT5 and up-regulation of Bcl-2. This analysis showed that STAT5 activation was entirely IL-7Rα Y449 dependent in all IL-7–responsive subsets (thymic DN, CD4 and CD8, and splenic CD4 and CD8 T cells) (Fig. 8 A). Surprisingly, two prosurvival members of the Bcl-2 family, Bcl-2 and Bcl-xL, were IL-7Rα Y449 independent (Fig. 8 B and unpublished data). Analysis of Bcl-2 regulation showed that IL-7Rα449F DN thymocytes had decreased levels of Bcl-2 after 24 h ex vivo culture compared with WT and that they were severely impaired in their ability to up-regulate Bcl-2 in response to IL-7 (Fig. 8 B). However, the remaining thymocyte subsets and peripheral T cells showed significant induction of Bcl-2 expression at the same time points (Fig. 8 B) leading to the conclusion that IL-7–mediated Bcl-2 regulation is mostly Y449 independent. Our findings support the hypothesis that IL-7–induced Bcl-2 is important during the DN stages of development to impart survival signals that allow differentiation events to occur (26, 27), but importantly, refutes the notion that Bcl-2 is sufficient in IL-7Rα–mediated events in peripheral T cells, particularly maintenance of CD8 memory T cells.
Figure 8.
IL-7Rα449F mutation abrogates activation of STAT5 but Bcl-2 up-regulation is Y449 independent. (A) Intracellular flow cytometry of WT and IL-7Rα449F lymphocytes stimulated ex vivo showed that activation of the STAT5 pathway is dependent on IL-7Rα Y449 in both the thymus and periphery. Isotype antibody, gray filled trace; untreated, dashed trace; +IL-7, solid trace. (B) Bcl-2 up-regulation is IL-7Rα Y449 independent in all thymic subsets and peripheral T cells except DN. Bar charts shown represent WT −IL-7, unfilled bars; WT +IL-7, black bars; IL-7Rα449F –IL-7, gray bars; IL-7Rα449F +IL-7, hatched bars. All experiments are representative of at least three mice for each genotype and two independent experiments.
DISCUSSION
Existing knockout models have severe limitations for discerning the requirement of IL-7Rα–induced signaling pathways downstream of T cell function in the periphery caused by secondary effects (1, 3, 38, 39). We have established a novel model, the IL-7Rα449F knock-in mouse, which allows analysis of development and function in T and B cells in the absence of IL-7Rα signaling from the Y449 residue. IL-7Rα Y449 was chosen for site-directed mutagenesis because previous work had linked activation of the PI3 kinase in B cells (30), human thymocytes (31), a human T lymphoblastoid cell line (32), and more recently, the STAT5 pathway to this residue (29). Similar to what is seen in IL-7Rα−/− mice, our analysis reveals that Y449 signaling is important in the early stages of T cell development. However, unlike IL-7Rα−/− mice, there is partial rescue of T cell numbers at later stages of thymic development. This novel aspect of the IL-7Rα449F mouse model means that in contrast with IL-7Rα−/− mice, IL-7Rα449F peripheral T cells are present at numbers amenable to further analysis.
The defect seen in the number of ETPs detected in IL-7Rα449F relative to that of WT suggests a role for IL-7Rα signaling at the earliest stages of T cell development. This is seen despite the lack of IL-7Rα on the surface of ETPs (40) and is thus suggestive of either defective trafficking of progenitors into the thymus or a survival defect in the circulating Lin−sca1+c-kit+ progenitor. Although a small population of circulating Lin−c-kit−B220+CD19−CD44hi cells has been reported to have T cell precursor potential (41), this elusive population (42) has not been quantified in the IL-7Rα449F mouse. Because the bone marrow HSC and post-HSC compartments are normal in both IL-7Rα449F and IL-7Rα−/− animals, it is likely that the IL-7Rα449F mutation affects early differentiation or survival events that account for the decrease in resident thymic progenitors. The difference in ETP development between IL-7Rα449F and IL-7Rα−/− also indicates signaling events that occur independently of the Y449 residue are sufficient to support some ETP development. Moreover, Y449-independent signals are sufficient to enable further T cell development because the 20-fold decrease in ETPs is overcome and thymocyte and peripheral T cell numbers are decreased by only fourfold. Our data also suggests that positive selection occurs in the absence of IL-7Rα–dependent signals because cells that successfully transition from DNIV to DP have normal frequencies of cells downregulating HSA and upregulating CD69. Together with our data showing IL-7Rα Y449–dependent Bcl-2 up-regulation in the DN compartment, these findings strongly support the hypothesis that the primary role of IL-7Rα in early thymocyte development is providing Bcl-2–mediated protection from apoptosis. Although the IL-7Rα subunit is also shared with the thymic stromal lymphopoietin (TSLP) receptor to mediate TSLP signaling (43), it does not appear that the effects of IL-7Rα449F disruption are TSLP mediated because TSLP and TSLP receptor knockouts show no major B or T cell development defects (44, 45).
The limited defects we found in the IL-7Rα449F mice enabled us to expand on the role of IL-7Rα in the development and function of peripheral T cells as established by the IL-7−/− and IL-7Rα−/− mouse models and other indirect methods of blocking IL-7Rα function. For example, studies using adoptive transfer systems and/or neutralizing antibodies to IL-7Rα have shown that IL-7 plays a role in HP of naive T cells (9, 17) and is essential for survival of memory CD4 (14) and CD8 (9) T cells. Previous studies have indicated that the transcription factor STAT5 is involved in HP (46, 47). Our study supports these findings and provides a direct link between IL-7Rα Y449–mediated STAT5 activation and efficient homeostatic proliferation. The pool of proliferating IL-7Rα449F CD8 T cells are IL-7Rα independent and are likely memory phenotype cells responding to IL-15 signaling. Interestingly, IL-7Rα449F T cells have attenuated responses to low dose α-CD3 and anti-CD28 stimulation, suggesting possible integration of TCR and IL-7Rα downstream signaling. The decreased proliferation elicited by low levels of TCR signaling may contribute to impaired HP since previous work has suggested that IL-7 and TCR signaling synergize to promote optimal HP (17).
Most importantly, our data is at odds with the hypothesis that regulation of Bcl-2 is sufficient for maintenance of memory CD8 T cells. Previously, an adoptive transfer experiment of IL-7Rα−/− OT-I transgenic TCR CD8 T cells concluded that the defect in maintenance of memory CD8 T cells in this system was the decreased level of prosurvival Bcl-2 in these cells (9). However, IL-7Rα449F CD8 memory T cells express normal levels of Bcl-2 yet do not demonstrate long-term maintenance. Expression of Bcl-2 is not caused by antigen stimulus because the same frequency of unstimulated controls express similar levels of Bcl-2. These data indicate two possible mechanisms to explain the defect of IL-7Rα449F CD8 memory T cells: fewer T cells from the primary response could be recruited to memory during the immune contraction phase, or if contraction is normal, the memory cells could be lacking a survival signal other than Bcl-2, such as Bcl-xL or Mcl-1.
Although the original pool of effector CD8 T cells is the same size as WT controls, by day 45 the IL-7Rα449F pool of CD8 memory T cells is significantly smaller and continues to decrease over time. These cells retain the ability to expand upon secondary challenge in vivo, indicating that although CD4 T cells do not generate a primary response their presence is sufficient to provide the “help” signals necessary for reexpansion. This is further supported by our data showing that even in the presence of WT CD4 T cells, a smaller proportion of IL-7Rα449F effector CD8 T cells are maintained to memory stage than WT cells generated in the same host. Finally, this is in agreement with the finding that CD4 help after acute infection is antigen independent (48). Nonetheless, the decrease in cell numbers or the absence of IL-7Rα Y449–dependent signals compromises functional efficacy of CD8 memory T cells. When IL-7Rα449F mice were challenged with a homologous prime/boost regimen with a more virulent L. monocytogenes expressing gp33 of lymphocytic choriomeningitis virus, IL-7Rα449F CD8 memory T cells were unable to provide protective immunity (unpublished data).
Our in vivo analyses extend upon a report describing the effect of IL-7Rα Y449F mutation (29). In agreement with our findings in primary cells, that study showed that activation of STAT5 is Y449 dependent. However, in contrast to the suggestion that IL-7–mediated Bcl-2 regulation is also Y449 dependent, our in vivo model shows that loss of Bcl-2 up-regulation is dramatic in DN thymocytes but is mostly restored at later stages of development in the IL-7Rα449F mouse. Moreover, we have found that IL-7 does not induce PI3 kinase activation in murine primary T cells as measured by Akt phosphorylation. This is in contrast to B cells and human T cells (for review see reference 49) but is in keeping with findings in the CT6 murine T cell line (50). Our data suggests that IL-7 induction of Bcl-2 is STAT5 and Akt independent.
In conclusion, we have characterized a novel IL-7Rα mutant mouse that has provided insight into the role of IL-7 signaling during early stages of T cell development and function of peripheral T cells. We show that IL-7Rα Y449–dependent signals such as STAT5 are required for accumulation of ETPs in the thymus. Interestingly, we also demonstrate a functional defect in the ability of IL-7Rα449F CD4 T cells to generate a primary response to infection with rLM-SIY. This suggests a previously underappreciated role for IL-7 signaling in either TCR repertoire selection or for acquiring effector function. In contrast, IL-7Rα449F CD8 T cells undergo vigorous expansion at acute stages of infection, but a defect becomes apparent in the maintenance of these cells to memory stage. The loss of antigen-specific CD8 memory T cells despite continued Bcl-2 expression disputes a sufficient role for Bcl-2 in IL-7–mediated events in peripheral T cells (9). This suggests that IL-7–induced survival of CD8 memory T cells requires more than Bcl-2 expression and is being investigated further.
MATERIALS AND METHODS
Mice.
Animals were housed at the University of British Columbia, Microbiology and Immunology Department Animal Facility in accordance with University of British Columbia Animal Care and Biosafety Committee certificates. C57BL/6, B6.SJL-Ptprca Pepcb (BoyJ), B6.PL-Thy1a/CyJ (Thy1.1+), and IL-7Rα−/− mice were obtained from The Jackson Laboratory.
IL-7Rα449F mice generation.
IL-7Rα449F mice were generated by homologous recombination as detailed in Supplemental Materials and methods (available at http://www.jem.org/cgi/content/org/jem.20061871/DC1). A replacement vector was generated in pKSloxPNT (51) (provided by Robert Farese, Gladstone Institute of Cardiovascular Disease, University of California, San Francisco, CA) that substituted exons 6–8 encoding the transmembrane and cytoplasmic domains of IL-7Rα with a partial cDNA (nucleotides 707–1,398) encoding a Y-to-F mutation at residue 449 and an SV40 polyadenylation signal. The targeted locus would retain regulation by the endogenous promoter. RF8 ES cells (129/SvJae origin) (52) were electroporated with linearized vector, selected, and targeted ES clones were identified by genomic Southern analysis (see Supplemental Materials and methods). Two out of three targeted clones were transiently transfected with MC-Cre (provided by Shinya Yamanaka, Institute for Frontier Medical Sciences, Kyoto University, Kyoto, Japan) to delete the loxP-flanked NeoR cassette and subclones verified as described (see Supplemental Materials and methods). IL-7Rα+/449F founders were generated by standard procedures and backcrossed at least six generations with C57BL/6 mice. PCR genotyping was performed with primers A (5′ TCTTCCTGAACAGCCAG), B (5′ TCTCTTCTGTGAGCTACGG), and C (5′ CCCTGAACCTGAAACATAAA) for 30 cycles each of 30 s at 94°C, 30 s at 60°C, and 1 min at 72°C. Primers A and B amplify a 489-bp fragment from the WT allele, whereas primers A and C amplify a 595-bp fragment from the knock-in allele.
Antibodies.
FITC, PE, CyC, APC, Alexa 647, APC Cy7, PE Cy7, and biotinylated antibodies were obtained from BD Biosciences (anti-γδ TCR, NK1.1, B220, Gr-1, Mac-1, CD3ɛ, CD4, CD8α, CD25, CD44, CD69, CD90.1, CD90.2, c-kit, IL-2/15Rβ, γc, HSA, Sca-1, TCR-β, Bcl-2, phospho-STAT5, BrdU, and H-2Kb–Ig recombinant fusion protein, and mouse DimerXI) or eBioscience (anti-CD4, CD8α, CD45.2, and IL-7Rα). Unlabeled antibodies were obtained from Cell Signaling Technology (anti-Akt, phospho-Akt Ser473, phospho-STAT5, phospho-Erk, Erk, and p38 MAPK), BD Biosciences (anti-phospho p38 MAPK), Santa Cruz Biotechnology, Inc. (anti–IL-7Rα), and Sigma-Aldrich (anti–α-tubulin). Rabbit polyclonal anti-STAT5A and STAT5B were a gift from Dr. Lothar Hennighausen (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD). Fluorescent secondary antibodies used in the Odyssey Infrared Imaging System (LiCOR) were obtained from Rockland Immunochemicals (goat anti–rabbit IgG IRDye800) and Invitrogen (goat anti–mouse IgG Alexa 680). Horseradish peroxidase–conjugated secondary antibodies used in chemiluminescent immunoblotting were from GE Healthcare.
Flow cytometry.
Surface staining for stem cell and lymphocyte characterization was performed according to standard procedures. For analysis of phospho-STAT5 activation, cells were acid stripped (10 mM Na citrate, pH 4, 140 mM NaCl) and starved for 4 h in serum-free RPMI + 1% BSA. Cells were stimulated by addition of 25 ng/ml recombinant murine IL-7 (Chemicon) for 10 min at 37°C in serum-free media. Cells were fixed for 10 min in 1% PFA at RT and permeabilized in 100% ice-cold methanol. Cells were then stained for detection of phospho-STAT5, CD4, and CD8. For detection of Bcl-2 up-regulation, cells were cytokine stripped and serum starved as described for phospho-STAT5, and stimulated with IL-7 for 20 h at 37°C. Cells were fixed and permeablized for 1 h at RT in 1% PFA, 0.1% Tween-20 and stained for surface markers and intracellular Bcl-2. Samples were collected on either a FACSCalibur or LSRII (BD Biosciences), and data were analyzed with FlowJo software (Tree Star).
Cell cycle analysis.
Two doses of 1 mg BrdU were delivered i.p. to WT and IL-7Rα449F mice at 4-h intervals. 20 h after the second BrdU dose, thymi were harvested and stained for surface markers (for DN, α-CD25, CD44, and lineage cocktail α-CD3, CD4, CD8, γδ TCR, Gr-1, Mac1 B220; for DP, α-CD4, CD8). Cells were then treated according to manufacturer's instructions for detection of BrdU (BD Biosciences).
Western blotting.
Samples were lysed in lysis buffer (10 mM Tris-HCl, pH 7.5, 5 mM EDTA, 1% Triton X-100), and cleared lysates were quantified and normalized for total protein level. For immunoblot analysis of IL-7Rα expression, total thymocyte lysates were resolved by SDS-PAGE, and proteins were electrophoretically transferred to nitrocellulose. A polyclonal anti–IL-7Rα from Santa Cruz Biotechnology, Inc. (sc-662) was used for detection and visualized with donkey anti–rabbit HRP and SuperSignal West Pico ECL reagents (Pierce Chemical Co.). For analysis of signaling pathways, WT and IL-7Rα449F CD8 T cells were cultured for 48 h at 106 cells/ml in the presence of 2 μg/ml ConA in complete media, then maintained in 20 U/ml IL-2 for 3 d, and finally, cytokine stripped and serum starved for 4 h. Cells were stimulated with 25 ng/ml IL-7, 1 μg soluble α-CD3, or 10 nM IL-2. Western blots were probed for detection of phosphorylated and total levels of Akt, p38 MAPK, Erk, and STAT5 using the LiCor Odyssey imaging system.
Homeostatic proliferation.
Spleens were collected from mice and prepared as single cell suspensions. Red blood cells were lysed using ACK lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 100 μM EDTA). Lymphocytes were B cell depleted using sheep anti–mouse IgG DynaBeads (Invitrogen). Purified cells were CFSE labeled as previously described (53). T cell subsets were quantified by flow cytometry, and 5 × 106 T cells were transferred i.v. to CD45.1 congenic mice that either had or had not received a sublethal dose of irradiation the previous day (600 rads). After 7 d, spleens of recipient mice were harvested and stained for CD45.2, CD4, and CD8α.
Competitive repopulation.
Bone marrow cells were isolated from the femurs of Thy1.1 and IL-7Rα449F mice. After red blood cell lysis, 2 × 106 whole bone marrow cells from Thy1.1 (CD45.2+, CD90.1+) and IL-7Rα449F (CD45.2+, CD90.2+) donors were adoptively transferred into lethally irradiated (1200 rads) BoyJ (CD45.1+) congenic recipients. Recipient mice received Biosol (neomycin sulfate) at 2 mg/ml in their water the night before irradiation and for the next 2 wk. At 4 wk after injection, recipient thymuses were harvested and stained for CD45.2, CD90.1, CD90.2, CD4, and CD8α.
In vitro TCR stimulation assay.
Peripheral T cells were purified from the spleens of WT and IL-7Rα449F mice using α-CD4 and α-CD8 MACS beads (Miltenyi Biotec) according to manufacturer's instructions. Purified, CFSE-labeled CD4 and CD8 WT and IL-7Rα449F T cells were cultured in vitro for 3 d in RPMI + 10% FBS in the presence of 1 μg α-CD28 and the indicated doses of plate-bound α-CD3. Cells were harvested, stained for CD4 and CD8, and dilution of CFSE was monitored by cytometry.
L. monocytogenes infection and response assays.
Naive C57BL/6 and IL-7Rα449F mice were injected intravenously with 105 CFU or previously immunized mice infected with 106 CFU of rLM-SIY in PBS. At appropriate time points after infection, spleens were collected, homogenized to single cell suspension, red blood cells lysed, and the cells restimulated in vitro with epitope-specific peptides for 5 h at 37°C in the presence of 1 μl/106 cells Golgi plug (BD Biosciences). The CD4-specific LLO peptide was added at 5 μM and the CD8-specific SIY peptide at 1 μM in Iscove's media + 10% FCS. After stimulation, cells were fixed and permeabilized in 2% PFA, 0.2% Tween-20 for 20 min at RT. Cells were then stained for CD4, CD8α, and IFN-γ. Antigen-specific cells were designated as CD4+ or CD8+ cells that expressed IFN-γ after in vitro challenge with antigenic peptides. SIY peptide loading of the BD Biosciences MHC I–Ig dimer fusion protein was performed according to manufacturer's instructions with a 40-M excess of peptide. For flow cytometric analysis of SIY-specific CD8 T cells using peptide loaded dimer, 0.5 μg dimer/2 × 106 cells was used.
For adoptive transfer experiments, WT T cells were purified from Thy1.1 mice by B cell depletion, and 107 T cells were injected intravenously into C57BL/6 or IL-7Rα449F recipients. The next day, recipient mice were infected with 105 CFU of rLM-SIY, and analysis was performed as outlined in the previous paragraph (with addition of anti-CD90.1) at 7 d after infection. Analysis of Bcl-2 levels and BrdU incorporation was performed with or without peptide stimulation and without IL-7 stimulation. Bcl-2 intracellular staining was performed as described previously. For cell cycle analysis, mice were given a single 2-mg dose of BrdU i.p. and then fed BrdU in their water for 8 d (0.8 mg/ml). Staining was performed according to manufacturer's instructions (BD Sciences).
Statistical analysis.
All data are presented as mean ± SEM and analyzed by Student's t test. Significance was set at p-value of <0.05.
Online supplemental material.
Fig. S1 shows genomic Southern blot analysis of ES clones with knock-in targeted IL-7Rα loci. Fig. S2 shows that IL-7Rα449F peripheral T cells have largely unperturbed γc and IL-2/15Rβ receptor subunits. Supplemental Materials and methods provides details of the knock-in targeting of the IL-7Rα locus. Online supplemental material available at http://www.jem.org/cgi/content/org/jem.20061871/DC1.
Acknowledgments
We thank Robert Farese and Stephen Young for assisting us with ES cell manipulation and microinjection, and John Carrol and Gladstone Graphics department for their services. We thank Ken Harder and Pauline Johnson for critical reading of the manuscript and Warner Greene for his support.
This work was supported by the National Institutes of Health (GM54351 to M.A. Goldsmith), the Canadian Institutes of Health Research (MOP-67005 to N. Abraham), Canadian Foundation of Innovation, and the BC Knowledge Development Fund. L.C. Osborne was supported by a Michael Smith Foundation for Health Research Junior Graduate scholarship and the Canadian Institutes of Health Research/Michael Smith Foundation for Health Research Strategic Training Program in Transplantation Research.
The authors have no conflicting financial interests.
Abbreviations used: γc, gamma common chain; DN, double negative; DP, double positive; ES, embryonic stem; ETP, early thymic progenitor; F, phenylalanine; HP, homeostatic proliferation; HSA, heat stable antigen; HSC, hematopoietic stem cell; LLO, listeriolysin O; PI3, phosphatidylinositol-3; SP, single positive; TSLP, thymic stromal lymphopoietin; Y, tyrosine.
References
- 1.Peschon, J.J., P.J. Morrissey, K.H. Grabstein, F.J. Ramsdell, E. Maraskovsky, B.C. Gliniak, L.S. Park, S.F. Ziegler, D.E. Williams, C.B. Ware, et al. 1994. Early lymphocyte expansion is severely impaired in interleukin 7 receptor–deficient mice. J. Exp. Med. 180:1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Freeden-Jeffry, U., P. Vierira, L.A. Lucian, T. McNeil, S.E.G. Burdach, and R. Murray. 1995. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181:1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maraskovsky, E., M. Teepe, P.J. Morrissey, S. Braddy, R.E. Miller, D.H. Lynch, and J.J. Peschon. 1996. Impaired survival and proliferation in IL-7 receptor-deficient peripheral T cells. J. Immunol. 157:5315–5323. [PubMed] [Google Scholar]
- 4.Puel, A., S.F. Ziegler, R.H. Buckley, and W.J. Leonard. 1998. Defective IL7R expression in T(-)B(+)NK(+) severe combined imunodeficiency. Nat. Genet. 20:394–397. [DOI] [PubMed] [Google Scholar]
- 5.Hammarlund, E., M.W. Lewis, S.G. Hansen, L.I. Strelow, J.A. Nelson, G.J. Sexton, J.M. Hanifin, and M.K. Slifka. 2003. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9:1131–1137. [DOI] [PubMed] [Google Scholar]
- 6.Becker, T.C., E.J. Wherry, D. Boone, K. Murali-Krishna, R. Antia, A. Ma, and R. Ahmed. 2002. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 195:1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldrath, A.W., P.V. Sivakumar, M. Glaccum, M.K. Kennedy, M.J. Bevan, C. Benoist, D. Mathis, and E.A. Butz. 2002. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 195:1515–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schluns, K.S., K. Williams, A. Ma, X.X. Zheng, and L. Lefrancois. 2002. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 168:4827–4831. [DOI] [PubMed] [Google Scholar]
- 9.Schluns, K.S., W.C. Kieper, S.C. Jameson, and L. Lefrancois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426–432. [DOI] [PubMed] [Google Scholar]
- 10.Goldrath, A.W., and M.J. Bevan. 1999. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 11:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kieper, W.C., and S.C. Jameson. 1999. Homeostatic expansion and phenotypic conversion of naive T cells in response to self peptide/MHC ligands. Proc. Natl. Acad. Sci. USA. 96:13306–13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marrack, P., J. Bender, D. Hildeman, M. Jordan, T. Mitchell, M. Murakami, A. Sakamoto, B.C. Schaefer, B. Swanson, and J. Kappler. 2000. Homeostasis of alpha beta TCR+ T cells. Nat. Immunol. 1:107–111. [DOI] [PubMed] [Google Scholar]
- 13.Surh, C.D., and J. Sprent. 2005. Regulation of mature T cell homeostasis. Semin. Immunol. 17:183–191. [DOI] [PubMed] [Google Scholar]
- 14.Seddon, B., P. Tomlinson, and R. Zamoyska. 2003. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat. Immunol. 4:680–686. [DOI] [PubMed] [Google Scholar]
- 15.Tan, J.T., B. Ernst, W.C. Kieper, E. LeRogy, J. Sprent, and C.D. Surh. 2002. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 195:1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vivien, L., C. Benoist, and D. Mathis. 2001. T lymphocytes need IL-7 but not IL-4 or IL-6 to survive in vivo. Int. Immunol. 13:763–768. [DOI] [PubMed] [Google Scholar]
- 17.Seddon, B., and R. Zamoyska. 2002. TCR and IL-7 receptor signals can operate independently or synergize to promote lymphopenia-induced expansion of naive T cells. J. Immunol. 169:3752–3759. [DOI] [PubMed] [Google Scholar]
- 18.Surh, C.D., and J. Sprent. 2002. Regulation of naive and memory T-cell homeostasis. Microbes Infect. 4:51–56. [DOI] [PubMed] [Google Scholar]
- 19.Judge, A.D., X. Zhang, H. Fujii, C.D. Surh, and J. Sprent. 2002. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8+ T cells. J. Exp. Med. 196:935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kieper, W.C., J.T. Tan, B. Bondi-Boyd, L. Gapin, J. Sprent, R. Ceredig, and C.D. Surh. 2002. Overexpression of interleukin (IL)-7 leads to IL-15 independent generation of memory phenotype CD8+ T cells. J. Exp. Med. 195:1533–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton, S.E., M.C. Wolkers, S.P. Schoenberger, and S.C. Jameson. 2006. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat. Immunol. 7:475–481. [DOI] [PubMed] [Google Scholar]
- 22.Fry, T.J., and C.L. Mackall. 2002. Interleukin-7: from bench to clinic. Blood. 99:3892–3904. [DOI] [PubMed] [Google Scholar]
- 23.Sudo, T., S. Nishikawa, N. Ohno, N. Akiyama, M. Tamakoshi, H. Yoshida, and S. Nishikawa. 1993. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc. Natl. Acad. Sci. USA. 90:9125–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofmeister, R., A.R. Khaled, N. Benbernou, E. Rajnavolgyi, K. Muegge, and S.K. Durum. 1999. Interleukin-7: physiological roles and mechanisms of action. Cytokine Growth Factor Rev. 10:41–60. [DOI] [PubMed] [Google Scholar]
- 25.Van De Wiele, C.J., J.H. Marino, B.W. Murray, S.S. Vo, M.E. Whetsell, and T.K. Teague. 2004. Thymocytes between the β-selection and positive selection checkpoints are nonresponsive to IL-7 as assessed by STAT-5 phosphorylation. J. Immunol. 172:4235–4244. [DOI] [PubMed] [Google Scholar]
- 26.Akashi, K., M. Kondo, U. von Freeden-Jeffry, R. Murray, and I.L. Weissman. 1997. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 89:1033–1041. [DOI] [PubMed] [Google Scholar]
- 27.Maraskovsky, E., L.A. O'Reilly, M. Teepe, L.M. Corcoran, J.J. Peschon, and A. Strasser. 1997. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell. 89:1011–1019. [DOI] [PubMed] [Google Scholar]
- 28.Amos, C.L., A. Woetmann, M. Nielsen, C. Geisler, N. Odum, B.L. Brown, and P.R. Dobson. 1998. The role of caspase 3 and BclxL in the action of interleukin 7 (IL-7): a survival factor in activated human T cells. Cytokine. 10:662–668. [DOI] [PubMed] [Google Scholar]
- 29.Jiang, Q., W.Q. Li, R.R. Hofmeister, H.A. Young, D.R. Hodge, J.R. Keller, A.R. Khaled, and S.K. Durum. 2004. Distinct regions of the interleukin-7 receptor regulate different Bcl2 family members. Mol. Cell. Biol. 24:6501–6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dadi, H.K., S. Ke, and C.M. Roifman. 1993. Interleukin 7 receptor mediates the activation of phosphatidylinositol-3 kinase in human B-cell precursors. Biochem. Biophys. Res. Commun. 192:459–464. [DOI] [PubMed] [Google Scholar]
- 31.Pallard, C., A.P. Stegmann, T. van Kleffens, F. Smart, A. Venkitaraman, and H. Spits. 1999. Distinct roles of the phosphatidylinositol 3-kinase and STAT5 pathways in IL-7-mediated development of human thymocyte precursors. Immunity. 10:525–535. [DOI] [PubMed] [Google Scholar]
- 32.Venkitaraman, A.R., and R.J. Cowling. 1994. Interleukin-7 induces the association of phosphatidylinositol 3-kinase with the alpha chain of the interleukin-7 receptor. Eur. J. Immunol. 24:2168–2174. [DOI] [PubMed] [Google Scholar]
- 33.Bhandoola, A., A. Sambandam, D. Allman, A. Meraz, and B. Schwarz. 2003. Early T lineage progenitors: new insights, but old questions remain. J. Immunol. 171:5653–5658. [DOI] [PubMed] [Google Scholar]
- 34.Ye, S.K., Y. Agata, H.C. Lee, H. Kurooka, T. Kitamura, A. Shimizu, T. Honjo, and K. Ikuta. 2001. The IL-7 receptor controls the accessibility of the TCRgamma locus by Stat5 and histone acetylation. Immunity. 15:813–823. [DOI] [PubMed] [Google Scholar]
- 35.Ciofani, M., G.C. Knowles, D.L. Wiest, H. von Boehmer, and J.C. Zuniga-Pflucker. 2006. Stage-specific and differential Notch dependency at the alphabeta and gammadelta T lineage bifurcation. Immunity. 25:105–116. [DOI] [PubMed] [Google Scholar]
- 36.Kaech, S.M., J.T. Tan, E.J. Wherry, B.T. Konieczny, C.D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191–1198. [DOI] [PubMed] [Google Scholar]
- 37.Ku, C.C., M. Murakami, A. Sakamoto, J. Kappler, and P. Marrack. 2000. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 288:675–678. [DOI] [PubMed] [Google Scholar]
- 38.Yao, Z., Y. Cui, W.T. Watford, J.H. Bream, K. Yamaoka, B.D. Hissong, C. Li, S.K. Durum, Q. Jiang, A. Bhandoola, et al. 2006. Stat5a/b are essential for normal lymphoid development and differentiation. Proc. Natl. Acad. Sci. USA. 103:1000–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakayama, K., K. Nakayama, I. Negishi, K. Kuidi, Y. Shinkai, M.C. Louie, L.E. Fields, P.J. Lucas, V. Stewart, F.W. Alt, and D.Y. Loh. 1993. Disappearance of the lymphoid system in Bcl-2 homozygous mutant chimeric mice. Science. 261:1584–1588. [DOI] [PubMed] [Google Scholar]
- 40.Allman, D., A. Sambandam, S. Kim, J.P. Miller, A. Pagan, D. Well, A. Meraz, and A. Bhandoola. 2003. Thymopoiesis independent of common lymphoid progenitors. Nat. Immunol. 4:168–174. [DOI] [PubMed] [Google Scholar]
- 41.Martin, C.H., I. Aifantis, M.L. Scimone, U.H. von Andrian, B. Reizis, H. von Boehmer, and F. Gounari. 2003. Efficient thymic immigration of B220+ lymphoid-restricted bone marrow cells with T precursor potential. Nat. Immunol. 4:866–873. [DOI] [PubMed] [Google Scholar]
- 42.Schwarz, B.A., and A. Bhandoola. 2004. Circulating hematopoietic progenitors with T lineage potential. Nat. Immunol. 5:953–960. [DOI] [PubMed] [Google Scholar]
- 43.Levin, S.D., R.M. Koelling, S.L. Friend, D.E. Isaksen, S.F. Ziegler, R.M. Perlmutter, and A.G. Farr. 1999. Thymic stromal lymphopoietin: a cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J. Immunol. 162:677–683. [PubMed] [Google Scholar]
- 44.Carpino, N., W.E. Thierfelder, M.S. Chang, C. Saris, S.J. Turner, S.F. Ziegler, and J.N. Ihle. 2004. Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Mol. Cell. Biol. 24:2584–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Shami, A., R. Spolski, J. Kelly, T.J. Fry, P.L. Schwartzberg, A. Pandey, C.L. Mackall, and W.J. Leonard. 2004. A role for thymic stromal lymphopoietin in CD4+ T cell development. J. Exp. Med. 200:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burchill, M.A., C.A. Goetz, M. Prlic, J.J. O'Neill, I.R. Harmon, S.J. Bensinger, L.A. Turka, P. Brennan, S.C. Jameson, and M.A. Farrar. 2003. Distinct effects of STAT5 activation on CD4+ and CD8+ T cell homeostasis: development of CD4+CD25+ regulatory T cells versus CD8+ memory T cells. J. Immunol. 171:5853–5864. [DOI] [PubMed] [Google Scholar]
- 47.Kelly, J., R. Spolski, K. Imada, J. Bollenbacher, S. Lee, and W.J. Leonard. 2003. A role for Stat5 in CD8+ T cell homeostasis. J. Immunol. 170:210–217. [DOI] [PubMed] [Google Scholar]
- 48.Sun, J.C., M.A. Williams, and M.J. Bevan. 2004. CD4 T cells are required for the maintenance, not programming, of memory CD8 T cells after acute infection. Nat. Immunol. 5:927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang, Q., W.Q. Li, F.B. Aiello, R. Mazzucchelli, B. Asefa, A.R. Khaled, and S.K. Durum. 2005. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 16:513–533. [DOI] [PubMed] [Google Scholar]
- 50.Lali, F.V., J. Crawley, D.A. McCulloch, and B.M. Foxwell. 2004. A late, prolonged activation of the phosphatidylinositol 3-kinase pathway is required for T cell proliferation. J. Immunol. 172:3527–3534. [DOI] [PubMed] [Google Scholar]
- 51.Hanks, M., W. Wurst, L. Anson-Cartwright, A.B. Auerbach, and A.L. Joyner. 1995. Rescue of the En-1 mutant phenotype by replacement of En-1 with En-2. Science. 269:679–682. [DOI] [PubMed] [Google Scholar]
- 52.Meiner, V.L., S. Cases, H.M. Myers, E.R. Sande, S. Bellosta, M. Schambelan, R.E. Pitas, J. McGuire, J. Herz, and R.V. Farese Jr. 1996. Disruption of the acyl-CoA:cholesterol acyltransferase gene in mice: evidence suggesting multiple cholesterol esterification enzymes in mammals. Proc. Natl. Acad. Sci. USA. 93:14041–14046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Priatel, J.J., O. Utting, and H.S. Teh. 2001. TCR/self-antigen interactions drive double-negative T cell peripheral expansion and differentiation into suppressor cells. J. Immunol. 167:6188–6194. [DOI] [PubMed] [Google Scholar]