Abstract
The Asian snake Rhabdophis tigrinus possesses specialized defensive glands on its neck that contain steroidal toxins known as bufadienolides. We hypothesized that R. tigrinus does not synthesize these defensive steroids but instead sequesters the toxins from toads it consumes as prey. To test this hypothesis, we conducted chemical analyses on the glandular fluid from snakes collected in toad-free and toad-present localities. We also performed feeding experiments in which hatchling R. tigrinus were reared on controlled diets that either included or lacked toads. We demonstrate that the cardiotonic steroids in the nuchal glands of R. tigrinus are obtained from dietary toads. We further show that mothers containing high levels of bufadienolides can provision their offspring with toxins. Hatchlings had bufadienolides in their nuchal glands only if they were fed toads or were born to a dam with high concentrations of these compounds. Because geographic patterns in the availability of toxic prey are reflected in the chemical composition of the glandular fluid, snakes in toad-free regions are left undefended by steroidal toxins. Our findings confirm that the sequestration of dietary toxins underlies geographic variation in antipredatory behavior in this species and provide a unique example of sequestered defensive compounds in a specialized vertebrate structure.
Keywords: antipredator defense, bufadienolides, chemical defense, maternal provisioning, toads
Many invertebrates sequester dietary toxins for use in their own defense (1–4), including such classic cases as milkweed insects (4) and sea slugs (1). However, vertebrate examples of toxin sequestration, especially from vertebrate prey, are rare. The brilliantly colored neotropical poison frogs (Dendrobatidae), their Malagasy analogues (Mantellidae), and a few other anurans sequester defensive alkaloids from arthropods (5–11); the same is suspected for two genera of New Guinean birds (12). Accumulation of defensive toxins from vertebrate prey is known only from some populations of gartersnakes (Thamnophis sirtalis), which may incur a defensive advantage because of the transitory storage of tetrodotoxin from ingested newts (13).
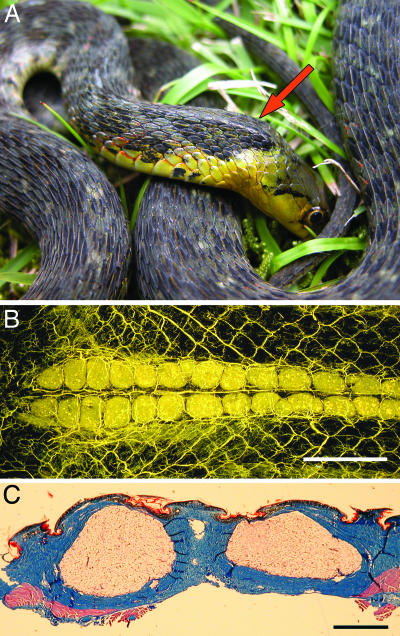
Rhabdophis tigrinus possesses a series of paired structures known as nuchal glands in the dorsal skin of the neck (14–17) (Fig. 1). The nuchal glands are associated with specific defensive behaviors that direct the dorsum of the neck toward an attacking predator (17–19). The fluid enclosed in the nuchal glands irritates mucous membranes (14) and contains cardiotonic steroids known as bufadienolides (20). Several lines of evidence suggest that R. tigrinus does not synthesize its defensive bufadienolides.R. tigrinus often consumes toads (Bufonidae) (21), which produce bufadienolides as major components of their skin secretions (22, 23). Histological and ultrastructural studies revealed that the nuchal glands of R. tigrinus lack secretory epithelia, and most of their cells lack secretory organelles, such as the Golgi apparatus and endoplasmic reticulum (K. Roberts and A.H.S., unpublished data). Instead, a dense network of capillaries in the nuchal glands suggests that bufadienolides are transported to the glands via the plasma (Fig. 1B). Furthermore, hatchlings of R. tigrinus that occupy a toad-free island (Kinkazan) use the nuchal glands in defensive displays less frequently and flee more often when threatened than individuals that occur sympatrically with toads (24). Such behavior suggests that Kinkazan snakes lack toxins in their nuchal glands. Based on these observations, we designed a series of experiments to test the hypothesis that R. tigrinus sequesters its defensive bufadienolides from ingested toads.
Fig. 1.
Nuchal glands of R. tigrinus. (A) Snake in typical defensive posture (“neck arch”), with head bent and dorsal skin of neck exposed to predator. Arrow indicates the ridge formed by the underlying nuchal glands. (B) Vascular cast of skin in ventral view, showing the dense capillary beds of the paired nuchal glands. Blood vessels have been filled with yellow latex, and the surrounding tissues have been cleared with methyl salicylate. Anterior is toward the left. (Scale bar: 5 mm.) (C) Transverse section through a pair of nuchal glands, showing the absence of a secretory epithelium, lumen, or duct. The blue tissue is dermal collagen, which forms a dense capsule around each gland. The glands empty by rupturing through the thin skin between adjacent scales. Trichrome stain was used. (Scale bar: 1 mm.)
Results
To determine whether a correlation exists between the distribution of toads and the presence of bufadienolides in R. tigrinus, we compared the compositions of nuchal gland fluid from adult snakes collected in three regions: the toad-free island of Kinkazan (Miyagi prefecture), the toad-rich island of Ishima (Tokushima prefecture), and various localities on Honshu (the main island of Japan) where toads occur. The presence of bufadienolides in nuchal gland fluid was assessed by using proton NMR (1H-NMR) spectroscopy of unfractionated samples (25) by identifying signals corresponding to the pyranone protons of bufadienolides. The presence of bufadienolides was corroborated in select samples by using high-performance liquid chromatography and mass spectroscopy (HPLC-MS). These methods revealed substantial variation in the quantity of bufadienolides in the nuchal gland fluid of snakes from different regions. Snakes from Ishima (n = 4), where toads are abundant, contained large quantities of bufadienolides, whereas snakes from Kinkazan (n = 3), where toads are absent, lacked bufadienolides completely. Snakes from various localities on Honshu (n = 15), sympatric with Japanese toads, possessed a wide range of bufadienolide concentrations.
We tested the sequestration hypothesis by rearing hatchling R. tigrinus on diets either containing or lacking toads. Our objectives were to assess whether unfed hatchlings contain bufadienolides in their nuchal glands and to determine whether hatchlings can sequester those toxins from ingested toads. We collected four gravid R. tigrinus from the Kyoto and Okayama prefectures on Honshu, where toads occur, and sampled the nuchal gland fluid of each for chemical analysis. We also collected glandular fluid from several newly hatched offspring from each clutch before rearing them on alternative diets of fish, non-bufonid (non-toad) frogs that lack bufadienolides, or North American toads, which contain bufadienolides. We then resampled the nuchal gland fluid of the hatchlings one or two more times. Each clutch consisted of 4–11 hatchlings.
Chemical analyses of prey extracts were performed by using 1H-NMR spectroscopy, and selected samples were analyzed further by using HPLC-MS. These analyses confirmed the presence of bufadienolides in the integumentary parotoid gland secretions of Bufo terrestris and Bufo fowleri and whole-skin extracts of juvenile Bufo quercicus that were used as prey for toad-fed R. tigrinus hatchlings [supporting information (SI) Fig. 6 A and B]. Major components of the bufadienolide mixtures produced by B. fowleri and B. terrestris, not previously analyzed in detail, were isolated by using HPLC and subsequently identified by using NMR spectroscopy and MS (Fig. 2 andSI Table 2). We also confirmed that bufadienolides were absent from the whole-body extracts of the fish (Pimephales promelas) and the skin extracts of the non-bufonid frogs (Spea multiplicata andScaphiopus holbrookii; Pelobatidae) that served as negative controls in the feeding studies (SI Fig. 6C).
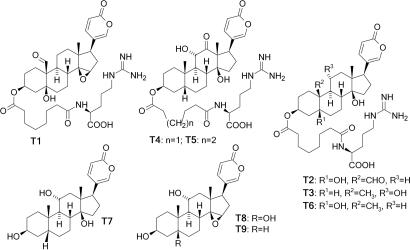
Fig. 2.
Bufadienolides from the toads B. fowleri and B. terrestris. Major components from B. fowleri include compounds T1, T2 (hellebritoxin), T3, T4, T5, and T6, whereas those in B. terrestris include T3, T6, T7 (gamabufotalin), T8, and T9 (11α-hydroxyresibufagenin). Compounds T1, T4, and T6 are new natural products.
Of the four dams (mothers) used in our experiment, two had no bufadienolides and one exhibited only trace amounts; the glandular fluid consisted primarily of water and lipids, with trace amounts of non-bufadienolide steroids (mainly cholesterol) and carbohydrates. The absence of substantial quantities of bufadienolides suggests that these dams had not consumed toads for a considerable time, if ever, before capture. None of the sampled offspring from these three dams exhibited bufadienolides in their nuchal gland fluid at hatching or after feeding on fish or pelobatid frogs (Fig. 3A). No other potentially defensive compounds, such as biogenic amines, were identified in their nuchal gland fluid, although these compounds are known from some pelobatids (22).
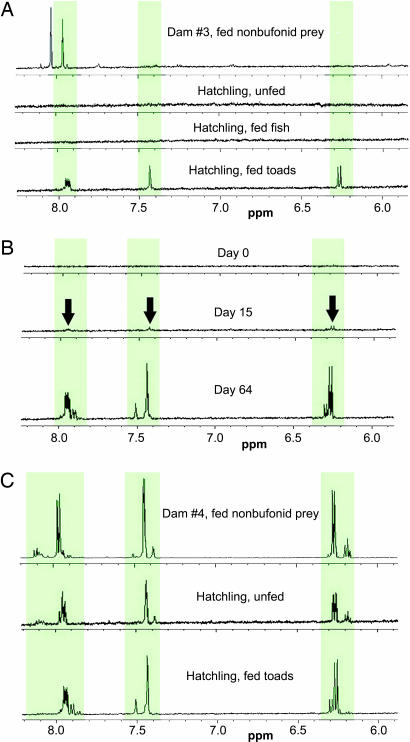
Fig. 3.
Aromatic region of 1H-NMR spectra of nuchal gland fluid from R. tigrinus. (A) Dam no. 3 and her hatchlings. The dam and her unfed and fish-fed hatchlings lacked bufadienolides (note the absence of peaks in the three regions diagnostic of bufadienolides, highlighted in green). The hatchlings only accumulated the toxins when fed toads. (B) A hatchling born to dam no. 3 that lacked bufadienolides at hatching (day 0) but accumulated increasing quantities as toads were consumed during the following 2 months. Arrows indicate the presence of small quantities of bufadienolides. (C) A chemically defended dam (no. 4) and her offspring, which were maternally provisioned with toxins.
In contrast, hatchlings from all three of these clutches rapidly and consistently accumulated bufadienolides in their nuchal glands when fed toads (Fig. 3A). For example, one hatchling that did not possess bufadienolides after feeding on fish for 8.5 weeks exhibited nearly 0.1 mg of these compounds in its nuchal gland fluid only 3 days after feeding on juvenile B. quercicus for 2 consecutive days. One hatchling that was sampled three times over 64 days continued to accumulate bufadienolides as additional toads were consumed (Fig. 3B). The results from these three clutches provide chemical evidence that dietary toxins are sequestered by R. tigrinus from toads.
The fourth dam from Honshu had very high concentrations of bufadienolides in her nuchal glands (Fig. 3C), presumably sequestered from Japanese toads consumed before capture. A sample of nuchal gland fluid from this dam contained >5 mg of bufadienolides. Correspondingly, her offspring possessed bufadienolides immediately upon hatching, in quantities of 0.1–1 mg per sampling (Fig. 3C). This finding indicates that chemically defended R. tigrinus can provision their hatchlings with bufadienolides, a pattern that we have recently observed in 10 additional clutches (unpublished data). The hatchlings from the fourth dam's clutch retained bufadienolides in their nuchal glands for at least 8.5 weeks, regardless of their diet in the laboratory. Additionally, the fact that individuals fed North American toads accumulated new types of bufadienolides that they did not possess upon hatching (Table 1) provided further evidence for sequestration of dietary toxins.
Table 1.
Bufadienolides in the nuchal gland fluid ofR. tigrinus
| Group | Clutch no. 1 (N = 11) | Clutch no. 2 (N = 4) | Clutch no. 3 (N = 8) | Clutch no. 4 (N = 10) |
|---|---|---|---|---|
| Dam, fed fish and frogs | None (n = 1)* | 6/7 (n = 1)† | None (n = 1)* | 5, 6/7, 8, 10, 11, 14, 15, 16 (n = 1)§ |
| Hatchlings, unfed | None (n = 3)* | None (n = 4)* | None (n = 3)* | 6/7, 8, 10, 14, 15, 16 (n = 3)‡ |
| Hatchlings, fed fish | None (n = 8)* | None (n = 1)* | None (n = 6)* | 6/7, 8, 10, 14, 15, 16 (n = 5)‡ |
| Hatchlings, fed frogs | None (n = 1)* | None (n = 1)* | None (n = 2)* | 6/7, 8, 10, 14, 15, 16 (n = 3)‡ |
| Hatchlings, fed toads | 1, 6/7, 8, 9, 10, 11, 12, 13, 17 (n = 2)† | 1, 6/7, 8, 9, 10, 11, 12, 13, 17 (n = 3)† | 1, 3, 4, 6/7, 8,9, 10, 11, 12, 13, 17 (n = 3)† | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 (n = 4)‡ |
Bufadienolides are identified by compound number (Fig. 5). The most abundant compound in each category is underlined. Bufadienolides 6 and 7 coelute from the HPCL column, so they could not always be distinguished. Total quantities of bufadienolides in individual samples are represented as follows:
∗, lack of bufadienolides;
†, 1–100 μ g;
‡, 0.1–1 mg; and
§, more than 5 mg.
N represents the total number of hatchlings per clutch. n represents the number of samples analyzed by 1H-NMR. Most hatchlings were sampled more than once over a 9-week period, to detect the effect of diet on nuchal gland composition.
To determine whether snakes from the toad-free island of Kinkazan are capable of sequestering bufadienolides and to obtain further evidence for maternal provisioning of bufadienolides, we conducted additional experiments involving six clutches each from Kinkazan and Ishima (unpublished data). The hatchlings from these 12 clutches were reared on controlled diets that either contained or lacked Japanese toads (Bufo japonicus), after which their nuchal gland fluid was sampled. As in the first experiment, the dams were fed only fish and/or non-bufonid frogs in the laboratory before oviposition; no information was available on the diets of these individuals before capture. However, we are confident that dams from Kinkazan had never consumed toads, whereas snakes from Ishima most likely preyed on the dense population of toads on that island.
These experiments revealed that unfed juvenile snakes from Kinkazan (n = 2) and those fed non-bufonid prey (n = 19) lacked bufadienolides in their nuchal glands. However, Kinkazan snakes sequestered bufadienolides when fed toads in the laboratory (n = 17). These results further support the sequestration hypothesis and demonstrate that R. tigrinus on Kinkazan have not lost the ability to sequester toxins. In contrast, all juvenile R. tigrinus from Ishima possessed bufadienolides in their nuchal glands regardless of their diet in the laboratory. The presence of large quantities of bufadienolides (typically 1–2 mg per sampling) in unfed juveniles (n = 6) and those fed non-bufonid prey (n = 17) suggests that maternal provisioning of defensive bufadienolides is common where toads are abundant. Furthermore, juveniles from Ishima that were fed toads possessed more types of bufadienolides than their unfed siblings or those fed non-bufonid prey.
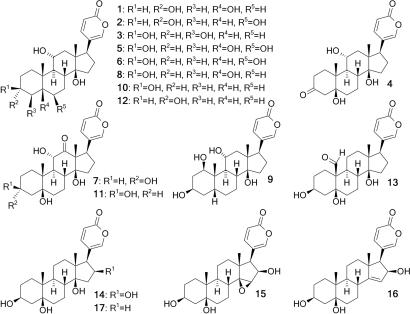
The chemical structures of the bufadienolides we detected in R. tigrinus were elucidated after HPLC fractionation of pooled samples of nuchal gland fluid from toad-fed hatchlings born to the chemically defended dam from Honshu (Fig. 4). Pure samples of individual bufadienolides were subsequently characterized by using MS and two-dimensional NMR spectroscopy. Seventeen bufadienolides were identified, six of which are new natural products (Fig. 5 and SI Tables 2 and 3). Eight of these bufadienolides (compounds 1–4, 9, 12, 13, and 17) were present only after the snakes had consumed North American toads (SI Fig. 7 and Table 1). At least eight bufadienolides were naturally present in the defended dam from Honshu, six of which were provisioned to her offspring in amounts large enough to be identified (SI Fig. 8 and Table 1). A summary of the distribution of bufadienolides among the four dams from Honshu and their offspring fed controlled diets is displayed in Table 1.
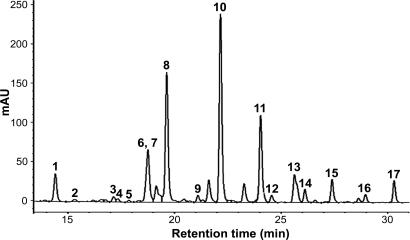
Fig. 4.
HPLC chromatogram of pooled samples of nuchal gland fluid from R. tigrinus hatchlings born to dam no. 4 that were fed toads for 34–64 days. The 17 bufadienolides we identified are indicated by number (see Fig. 5). mAU, milli-absorbance units at 280 nm.
Fig. 5.
Seventeen bufadienolides from the nuchal gland fluid of R. tigrinus. Compound 8 is 11α-hydroxytelocinobufagin, compound 10 is gamabufotalin, compound 13 is hellebrigenin, and compound 17 is telocinobufagenin. Compounds 2–6 and 9 are new natural products.
Discussion
The bufadienolides from nuchal gland fluid lacked the suberylarginine side-chain characteristic of bufotoxins, which are C3-acylated bufadienolides present as major components of the skin secretions of both American and Japanese toads (26, 27). The absence of bufotoxins in the nuchal gland fluid suggests that R. tigrinus either selectively takes up nonacylated bufadienolides from toads or, more likely, cleaves the suberylarginine side chains from bufotoxins after uptake. The mechanisms of bufadienolide uptake and storage in R. tigrinus have not yet been investigated. However, a preliminary analysis of plasma from one female revealed the presence of small amounts of two bufadienolides, suggesting that the dense capillary networks of the nuchal glands function in delivery of toxins.
Some bufadienolides, such as gamabufotalin (10 and T7), appear to be sequestered unaltered or could be derived from side-chain hydrolysis of bufotoxins (10 from T3; 13 from T2; 17 from T6). Most of the remaining bufadienolides in R. tigrinus resemble bufonid bufadienolides that have undergone additional hydroxylation after uptake by the snakes (Figs. 2 and 5). Hydroxylation of sequestered dietary toxins has been reported in predacious fireflies (28) and dendrobatid frogs (7). In dendrobatids, hydroxylation dramatically increases the toxicity of a sequestered alkaloid (7). The bufadienolides in R. tigrinus that have been studied for activity vary widely in their inhibition of the sodium-potassium pump and their positive inotropic action on heart muscle (29), which may be in part due to differences in hydroxylation among compounds. Hydroxylation may also influence the bioavailability of bufadienolides.
Our results provide chemical support for the hypothesis that R. tigrinus sequesters defensive bufadienolides from a dietary source. We found that when hatchling R. tigrinus lack bufadienolides, they can acquire those toxins from ingested toads. Juvenile R. tigrinus prey primarily on non-bufonid frogs (30) but also consume larval and juvenile toads, when such food is available (ref. 31 and unpublished data). Unfed hatchlings display higher tongue-flicking scores for bufonid extracts than for other prey extracts, demonstrating an innate preference for toads (32). The offspring of defended dams possessed bufadienolides at hatching, because of maternal provisioning of toxins sequestered by the dam from dietary toads. Such defended hatchlings can increase the diversity of bufadienolides in their arsenal by consuming toads that possess additional bufadienolide compounds. Subsequent experiments have confirmed the role of maternal provisioning and have demonstrated a strong positive relationship between the quantities of bufadienolides in dams and their offspring.
Our comparison of snakes from a toad-free island (Kinkazan) with those from a toad-rich island (Ishima) demonstrate the influence of local prey availability on defensive chemistry and behavior in R. tigrinus. We interpret the reliance on flight for defense in Kinkazan snakes (24) as an evolved response to the absence of toxic prey.
The accumulation of sequestered toxins in the specialized defensive structures of R. tigrinus is unique among terrestrial vertebrates. Our results not only document a rare case in which defensive toxins are acquired from vertebrate prey but also provide a compelling example of the geographic, ecological, and evolutionary interplay between diet and antipredator defense (33, 34). Furthermore, our findings suggest that maternal diet and provisioning may be important determinants of hatchling fitness in this chemically defended vertebrate.
Materials and Methods
Experimental Design and Sample Collection.
Four gravid female R. tigrinus were collected in the Kyoto and Okayama prefectures, Japan. They were fed only non-bufonid frogs (Rhacophorus arboreus and/or Rana nigromaculata) in captivity before being shipped to the United States along with their clutches in July 2004. Nuchal gland fluid was collected from the chemically defended dam (no. 4) in the U.S. after being fed goldfish (Carassius auratus); the other three dams (nos. 1–3) appeared too emaciated after oviposition to be subjected to sampling. Nuchal gland fluid was collected from these three dams postmortem, by which time they had been fed only fish and non-bufonid frogs in captivity.
To collect nuchal gland fluid, we placed a section of a laboratory tissue (Kimwipe; Kimberly–Clark, Dallas, TX) over the dorsal surface of the neck and gently squeezed until the glands ruptured through the skin. The tissue was then inserted into a vial of HPLC-grade methanol with forceps, sealed with a Teflon-lined cap, and stored at −20°C for later analysis. We changed gloves, rinsed the forceps in deionized water, and dried the forceps with a Kimwipe between each individual. At least one control vial (Kimwipe without glandular fluid, placed into methanol with forceps) was prepared near the end of the sampling sequence on each day that samples were collected.
The four clutches of eggs were incubated on moist vermiculite in a 30°C incubator until hatching, at which time a small volume of nuchal gland fluid was collected from three unfed hatchlings from each clutch. We were careful only to express a few glands, to ensure that we could resample these individuals at later dates, because nuchal glands do not appear to regenerate, at least initially, after they have ruptured. This limited the number of times an individual snake could be sampled. For most snakes, two or three samplings could be performed.
Sampled hatchlings were randomly assigned to different feeding groups. One group was fed only fish (P. promelas), another was fed non-bufonid frogs (juvenile Sc. holbrookii or metamorphic Sp. multiplicata), and the third group was fed toads (B. fowleri, B. quercicus, and/or B. terrestris). We used more than one species of Bufo as prey, because the availability of toads was limited. All individuals in the toad-fed group received metamorphic B. terrestris, until our supply was exhausted. We then fed this group juvenile B. fowleri, cut portions of previously frozen adult B. terrestris, and/or juvenile B. quercicus. Hatchlings that were unsampled in the naïve state were fed fish, toads, or both. Some individuals were switched from one of the non-toad-fed groups to the toad-fed group; nuchal gland fluid from these individuals was collected before and after the change in diet. After the snakes had fed for several days, we resampled their nuchal gland fluid. A few hatchlings survived long enough to allow three samples to be collected, but most died after the second sample was taken. Sampling of nuchal gland fluid was unlikely to have been a factor in the deaths of these snakes, which are notoriously difficult to maintain in captivity for extended periods, as either adults or young. Our animal use protocols were approved by the Old Dominion University Institutional Animal Care and Use Committee.
It is difficult to determine precisely the quantity of bufadienolides in sequential samples of nuchal gland fluid, because the fluid cannot be uniformly expressed from the glands. We attempted to standardize the volume of nuchal gland fluid collected at each sampling period, but the third sample obtained from several hatchlings yielded noticeably smaller volumes of fluid, because few remaining glands were available for expression. Therefore, the gradual accumulation of bufadienolides observed in one toad-fed hatchling over three sampling periods almost certainly represents a real phenomenon and not a sampling artifact.
NMR-Spectroscopic Analyses.
Samples of nuchal gland fluid were evaporated to dryness, reconstituted in deuterated methanol (CD3OD), and analyzed with 1H-NMR spectroscopy before fractionation, to determine the presence of bufadienolides and other compounds of interest (25). In cases where the identity of the NMR-spectroscopic signals observed could not be determined unambiguously on the basis of one-dimensional 1H-NMR spectra alone (for example, in cases of severe signal overlap in the aromatic region), additional phase-sensitive DQF-COSY spectra were acquired (25). NMR-spectroscopic analyses were performed with Unity INOVA 500-MHz and INOVA 600-MHz spectrometers (Varian, Palo Alto, CA), which were equipped with Oxford magnets (Oxford Instruments, Eynsham, Witney, Oxon, U.K.) and HCN or broadband probes.
For structural characterization and identification of isolated bufadienolides, a series of two-dimensional NMR spectra was acquired that included a phase-cycled phase-sensitive DQF-COSY spectrum (parameters: 600 ms acquisition time; 400–600 complex increments in F1; and 4, 8, or 16 scans per increment), a phase-sensitive nongradient heteronuclear multiple-quantum correlation spectrum, a magnitude-mode nongradient HMBC spectrum, and a phase-sensitive NOESY spectrum (using a mixing time of 600 ms).
Amounts of bufadienolides in the nuchal gland samples were determined by integration of the 1H-NMR signals that represent the pyranone protons and subsequent comparison of the respective integrals with those obtained from 1H-NMR spectra of a bufadienolide standard (telocinobufagin). By using DQF-COSY spectra, it was established that the 1H-NMR signals in the nuchal gland spectra that were used for integration indeed represented only pyranone protons and were not contaminated with signals derived from other structural features.
Isolation of Bufadienolides and HPLC-MS.
For isolation of bufadienolides from nuchal gland fluid samples and toad skin secretions, we used an Agilent (Santa Clara, CA) 1100 Series HPLC system equipped with a quaternary pump, a diode array detector, and autosampler. The samples were fractionated through a reversed-phase 25 cm × 10 mm Supelco (Bellefonte, PA) Discovery HS C18 column, and fractions of interest were collected with a Foxy 200 fraction collector (Teledyne Isco, Lincoln, NE). A solvent gradient system was used, starting with a 20:80 methanol:water mixture, the methanol content of which was increased linearly from 20% at three minutes to 100% at 25 min, by using a constant flow rate of 3.4 ml/min. The injection volumes of the samples ranged from 10 to 25 μl. HPLC-MS analyses were carried out by using a Quattro I tandem mass spectrometer (Micromass, Manchester, U.K.), operated in positive-ion electrospray mode and connected to the same Agilent 1100 Series HPLC system.
Analysis of Prey Items.
Samples of the prey items were analyzed for the presence of bufadienolides by using NMR spectroscopy and HPLC (see NMR-Spectroscopic Analyses and Isolation of Bufadienolides and HPLC-MS). For juvenile and adult toads, we collected skin secretion either by squeezing the parotoid glands directly onto a Kimwipe or by stimulating the skin with a transcutaneous amphibian stimulating device (35). We prepared whole-body extracts of the fish and whole-skin or whole-body extracts of the small frogs and toads. Surprisingly, bufadienolides were absent in the skin of metamorphic B. terrestris (SI Fig. 6B) (cf. ref. 36), which were fed to hatchling snakes in the toad-fed group. Later analyses of whole-body extracts revealed that most metamorphic B. terrestris lack bufadienolides altogether, although some have minute amounts. Almost all of the snakes in the toad-fed group were fed several species or ontogenetic stages of toads, so the lack of bufadienolides in metamorphic B. terrestris did not have a detrimental effect on our study.
Supplementary Material
Acknowledgments
We thank M. Hasegawa, Y. Kadota, E. Nagata, A. Nakadai, K. Mochida, M. Toda, T. L. Adams, J. F. Bolin, J. Bradford, M. Close, A. R. Lemmon, E. C. Moriarty Lemmon, J. M. Ray, B. A. Savitzky, and V. R. Townsend, Jr. for collecting animals in the field; S. J. Davis, D. Kuklock, G. T. Mueller, J. M. Ray, E. M. Schmutzler, K. Tanaka, and K. White for animal care; P. Weldon for early discussions; D. E. Sonenshine for early chemical assistance; K. A. Roberts for histological assistance; J. B. Grant for construction and advice on the transcutaneous amphibian stimulating device; T. F. Spande and J. W. Daly for supplying the telocinobufagin standard; and J. W. Daly, T. Eisner, V. R. Townsend, Jr., and V. C. Clark for comments on the manuscript. This work was supported by National Science Foundation Grants IBN-0429223 and IOB-0519458 (to A.H.S. and J.M.) and INT-9513100 (to G.M.B.); an Old Dominion University Dissertation Fellowship (D.A.H.); the Society for Integrative and Comparative Biology and Sigma Xi, The Scientific Research Society Grants-in-Aid of Research programs (to D.A.H.); a Kyoto University Grant for Biodiversity Research of the 21st Century COE A14 (to A.M. and the Department of Zoology, Kyoto Universtity); a Kyoto University Museum Visiting Faculty Award (to A.H.S.); the Japan Society for the Promotion of Science (A.M.); and the Fujiwara Natural History Foundation 10th Annual Grant for Scientific Research (to A.M.).
Abbreviations
- HPLC-MS
high-performance liquid chromatography and mass spectroscopy
- 1H-NMR
proton NMR.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610785104/DC1.
References
- 1.McPhail KL, Davies-Coleman MT, Starmer J. J Nat Prod. 2001;64:1183–1190. doi: 10.1021/np010085x. [DOI] [PubMed] [Google Scholar]
- 2.Duffey SS. Annu Rev Entomol. 1980;25:447–477. [Google Scholar]
- 3.Nishida R. Annu Rev Entomol. 2002;47:57–92. doi: 10.1146/annurev.ento.47.091201.145121. [DOI] [PubMed] [Google Scholar]
- 4.Brower LP. In: The Biology of Butterflies. Vane-Wright RI, Ackery PR, editors. London: Academic; 1984. pp. 109–134. [Google Scholar]
- 5.Clark VC, Raxworthy CJ, Rakotomalala V, Sierwald P, Fisher BL. Proc Natl Acad Sci USA. 2005;102:11617–11622. doi: 10.1073/pnas.0503502102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daly JW. J Nat Prod. 1998;61:162–172. doi: 10.1021/np970460e. [DOI] [PubMed] [Google Scholar]
- 7.Daly JW, Garraffo HM, Spande TF, Clark VC, Ma JM, Ziffer H, Cover JF., Jr Proc Natl Acad Sci USA. 2003;100:11092–11097. doi: 10.1073/pnas.1834430100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garraffo HM, Spande TF, Daly JW, Baldessari A, Gros EG. J Nat Prod. 1993;56:357–373. doi: 10.1021/np50093a008. [DOI] [PubMed] [Google Scholar]
- 9.Jones TH, Gorman JST, Snelling RR, Delabie JHC, Blum MS, Garraffo HM, Jain P, Daly JW, Spande TF. J Chem Ecol. 1999;25:1179–1193. [Google Scholar]
- 10.Saporito RA, Garraffo HM, Donnelly MA, Edwards AL, Longino JT, Daly JW. Proc Natl Acad Sci USA. 2004;101:8045–8050. doi: 10.1073/pnas.0402365101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spande TF, Jain P, Garraffo HM, Pannell LK, Yeh HJC, Daly JW. J Nat Prod. 1999;62:5–21. doi: 10.1021/np980298v. [DOI] [PubMed] [Google Scholar]
- 12.Dumbacher JP, Wako A, Derrickson SR, Samuelson A, Spande TF, Daly JW. Proc Natl Acad Sci USA. 2004;101:15857–15860. doi: 10.1073/pnas.0407197101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams BL, Brodie ED, Jr, Brodie ED., III J Chem Ecol. 2004;30:1901–1919. doi: 10.1023/b:joec.0000045585.77875.09. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura K. Mem Coll Sci Kyoto Imp Univ. 1935;10B:229–240. [Google Scholar]
- 15.Smith MA. Proc Zool Soc London B. 1938;107:575–583. pl I. [Google Scholar]
- 16.Toriba M, Sawai Y. In: Snakes of Medical Importance (Asia-Pacific Region) Gopalakrishnakone P, Chou LM, editors. Singapore: Nat Univ Singapore; 1990. pp. 323–347. [Google Scholar]
- 17.Fukada H. Snake Life History in Kyoto. Tokyo: Impact Shuppankai; 1992. [Google Scholar]
- 18.Mori A, Burghardt GM. Ethology. 2001;107:795–811. [Google Scholar]
- 19.Mori A, Layne D, Burghardt GM. Jpn J Herpetol. 1996;16:94–107. [Google Scholar]
- 20.Akizawa T, Yasuhara T, Kano R, Nakajima T. Biomed Res. 1985;6:437–441. [Google Scholar]
- 21.Mori A, Moriguchi H. Snake. 1988;20:98–113. [Google Scholar]
- 22.Erspamer V. In: Amphibian Biology: Vol 1, The Integument. Heatwole H, Barthalmus GT, Heatwole AY, editors. Chipping Norton: Surrey Beatty; 1994. pp. 178–350. [Google Scholar]
- 23.Porto AM, Baralle FE, Gros EG. J Steroid Biochem. 1972;3:11–17. doi: 10.1016/0022-4731(72)90006-4. [DOI] [PubMed] [Google Scholar]
- 24.Mori A, Burghardt GM. J Comp Psychol. 2000;114:408–413. doi: 10.1037/0735-7036.114.4.408. [DOI] [PubMed] [Google Scholar]
- 25.Taggi AE, Meinwald J, Schroeder FC. J Am Chem Soc. 2004;126:10364–10369. doi: 10.1021/ja047416n. [DOI] [PubMed] [Google Scholar]
- 26.Meyer K, Linde H. In: Venomous Animals and Their Venoms: Vol 2, Venomous Vertebrates. Bücherl W, Buckley EE, editors. New York: Academic; 1971. pp. 521–556. [Google Scholar]
- 27.Chen KK, Chen AL. J Pharmacol Exp Ther. 1933;49:526–542. [Google Scholar]
- 28.González A, Schroeder FC, Attygalle AB, Svatoš A, Meinwald J, Eisner T. Chemoecology. 1999;9:105–112. [Google Scholar]
- 29.Azuma H, Sekizaki S, Akizawa T, Yasuhara T, Nakajima T. J Pharm Pharmacol. 1986;38:388–390. doi: 10.1111/j.2042-7158.1986.tb04594.x. [DOI] [PubMed] [Google Scholar]
- 30.Fukada H. Bull Kyoto Gakugei Univ B. 1959;14:22–28. [Google Scholar]
- 31.Goris RC, Maeda N. A Guide to the Amphibians and Reptiles of Japan. Malabar, FL: Krieger; 2005. [Google Scholar]
- 32.Mori A. Bull Herpetol Soc Jpn. 2004;2004:29–33. [Google Scholar]
- 33.Thompson JN. The Geographic Mosaic of Coevolution. Chicago: Univ Chicago Press; 2005. [Google Scholar]
- 34.Brodie ED, Jr, Ridenhour BJ, Brodie ED., III Evolution (Lawrence, Kans) 2002;56:2067–2082. doi: 10.1111/j.0014-3820.2002.tb00132.x. [DOI] [PubMed] [Google Scholar]
- 35.Grant JB, Land B. Herpetol Rev. 2002;33:38–41. [Google Scholar]
- 36.Flier J, Edwards MW, Daly JW, Myers CW. Science. 1980;208:503–505. doi: 10.1126/science.6245447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.