Abstract
Preferential homing of naive lymphocytes to secondary lymphoid organs is thought to involve the action of chemokines, yet no chemokine has been shown to have either the expression pattern or the activities required to mediate this process. Here we show that a chemokine represented in the EST database, secondary lymphoid-tissue chemokine (SLC), is expressed in the high endothelial venules of lymph nodes and Peyer’s patches, in the T cell areas of spleen, lymph nodes, and Peyer’s patches, and in the lymphatic endothelium of multiple organs. SLC is a highly efficacious chemoattractant for lymphocytes with preferential activity toward naive T cells. Moreover, SLC induces firm adhesion of naive T lymphocytes via β2 integrin binding to the counter receptor, intercellular adhesion molecule-1, a necessary step for lymphocyte recruitment. SLC is the first chemokine demonstrated to have the characteristics required to mediate homing of lymphocytes to secondary lymphoid organs. In addition, the expression of SLC in lymphatic endothelium suggests that the migration of lymphocytes from tissues into efferent lymphatics may be an active process mediated by this molecule.
During immune surveillance, naive lymphocytes preferentially enter secondary lymphoid organs where they sample sequestered antigens before returning to the circulation. In lymph nodes and Peyer’s patches, they leave the blood by emigrating across specialized high endothelial venules (HEV) (1). In the spleen, which lacks HEV, they exit the blood in the marginal zone and move to the white pulp by a route that has not been clearly defined (2). After leaving the blood, naive B and T lymphocytes migrate to distinct compartments within lymphoid organs, follicles and T cell areas, respectively, where they encounter antigen-presenting dendritic cells. Those lymphocytes that are stimulated by antigen are retained and proliferate while the majority return to the circulation. In this manner, lymphocyte clones reactive to rare antigens are rapidly selected, stimulated to differentiate into effector or memory cells, and expanded. Unlike naive lymphocytes, memory lymphocytes migrate preferentially to peripheral nonlymphoid organs. While the migration of these cells from the blood has been extensively studied (3, 4), little is known about the mechanisms by which they return to the circulation.
Lymphocytes are thought to enter secondary lymphoid organs through a cascade of steps (5). In this model, cells initially tether and roll on endothelium through selectin-mediated interactions (step 1), then undergo a stimulated increase in integrin activity (step 2) that causes them to firmly adhere to endothelium through the binding of integrins to Ig superfamily adhesion molecules (step 3). Firmly attached cells then follow a chemoattractant gradient into tissues (step 4). The selectins and integrins required for the first and third steps in this process have been defined (3, 4). In step 1, L-selectin expressed on lymphocytes interacts with peripheral node addressin, a complex of glycoproteins that includes CD34 (6). During step 3, the lymphocyte integrin αLβ2 (LFA-1, CD11/CD18) mediates firm adhesion by binding to its HEV-expressed counter-receptors, intercellular adhesion molecule-1 (ICAM-1) or ICAM-2 (7, 8). In the case of lymphocyte homing to gut-associated lymphoid tissues (e.g., Peyer’s patches), there is the additional involvement of α4β7 (LPAM-1), which can mediate rolling and slowing of the lymphocyte through an interaction with MadCAM-1 on HEV (9, 10).
Remaining to be identified are the molecules that provide a stimulus for increased integrin adhesiveness (step 2 above) and serve as chemoattractants (step 4) during lymphocyte homing. Thus far the best candidates for performing these functions are chemokines, a rapidly growing family of small basic proteins that direct the migration of leukocytes (11, 12). Chemokines are related by primary structure and characteristic properties such as the ability to bind heparin. They can be divided into four groups based on the arrangement of their conserved cysteines. All chemokines thus far studied exert their effects by binding to seven-transmembrane-domain G-protein coupled receptors. Chemokines have been shown to activate β1 or β2 integrins on monocytes, neutrophils, and lymphocytes and to serve as chemoattractants for these cells during inflammation (13–15). Circumstantial evidence suggests that they may perform similar functions during lymphocyte homing. Treatment of lymphocytes with pertussis toxin inhibits both the stimulated increase in αLβ2 (LFA-1)-mediated adhesion to HEV and lymphocyte entry into secondary lymphoid organs, consistent with the involvement of a Gαi-linked chemokine receptor in these processes (16–18). Despite this evidence, no chemokine has yet been demonstrated to have the expression pattern or the activities required to mediate the homing of naive lymphocytes to secondary lymphoid organs (19).
Recently, several lymphocyte-specific chemokines have been described that are expressed in secondary lymphoid organs (20–22). Here we investigate one of these, secondary lymphoid-tissue chemokine (SLC), with respect to its possible involvement in lymphocyte homing. We demonstrate that SLC is expressed in HEV and in areas of T cell accumulation in spleen, Peyer’s patches, and lymph nodes. SLC is a highly efficacious chemoattractant for lymphocytes with preferential activity toward naive T cells. Finally, SLC induces firm adhesion of naive T lymphocytes via β2 integrin binding to the counter receptor, ICAM-1.
METHODS
Sequence Analysis.
Pattern searches of the National Center for Biotechnology Information (NCBI) sequence database with tfasta (36) retrieved related SLC expressed sequence tags (ESTs) from human and mouse. I.M.A.G.E. Consortium (Lawrence Livermore National Laboratory) cDNA clones 332031, 389013, and 503192 (37) were obtained from Genome Systems (St. Louis) as EcoRI-NotI inserts in the pT7T3-Pac vector and sequenced. The Institute for Genomic Research clone EST113564 (38) was obtained from American Type Culture Collection and processed similarly.
RNA Expression Studies.
For Northern analysis, mRNA from mouse tissues or purified cells was subjected to gel electrophoresis, transferred to Hybond membranes, and probed by using the randomly primed mouse SLC EST cDNA. A human multi-tissue dot blot (CLONTECH) was probed with the human SLC EST. For in situ hybridizations, paraffin sections (5 μm) from C57BL6 mice were deparaffinized, fixed in 4% paraformaldehyde, and treated with proteinase K. After washing in 0.5× standard saline citrate (1× = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7), the sections were covered with hybridization solution, prehybridized for 1–3 hr at 55°C, and hybridized overnight with sense or antisense 35S-labeled riboprobe transcribed from the full-length mouse SLC EST. After hybridization, sections were washed at high stringency, dehydrated, dipped in photographic emulsion NTB2 (Kodak), stored at 4°C for 2–8 weeks, developed, and counterstained with hematoxylin and eosin.
Production of Recombinant Proteins.
The full-length mouse and human SLC ESTs were cloned into the pVL1393 baculovirus transfer vector and cotransfected with BaculoGold (PharMingen) into SF9 cells according to the manufacturer’s instructions. For protein production, SF21 cells were infected at a multiplicity of infection of 10–20 and cultured in serum-free media for 60 hr. Conditioned media was cleared, loaded onto a HiTrap heparin affinity column (Pharmacia), and eluted with a 0.2–1 M NaCl gradient in 50 mM Hepes (pH 7.9). Fractions containing only SLC were pooled, concentrated on Centriplus-10 concentrators (Amicon), and dialyzed against PBS.
Chemotaxis.
Mouse lymphocytes, obtained by passing teased spleen and lymph node cells through a 70-μm screen, were resuspended in RPMI 1640 medium containing 5% fetal calf serum, incubated for 1 hr at 37°C, and subjected to a chemotactic assay (25). For the assay, 106 cells in 100 μl medium were added to the top chamber of a 6.5-mm diameter, 5 μm pore polycarbonate Transwell insert (Costar, Cambridge, MA) and incubated in triplicate with the indicated concentrations of SLC in the bottom chamber for 3 hr. Cells migrating to the bottom chamber were resuspended, stained with antibodies to cell surface markers (CD4, CD8, B220, CD44, and L-selectin) and subjected to flow cytometric analysis and counted with a FACScan (Becton Dickinson). A 1:20 dilution of input cells was similarly analyzed. In some studies, cells were pre incubated with 100 ng/ml pertussis toxin (List Biological Laboratories, Campbell, CA) for 2 hr at 37°C. Mouse peripheral blood lymphocytes were obtained by erythrocyte lysis of whole blood, and assayed as above.
Controlled Detachment Adhesion Assay.
Recombinant soluble ICAM-1-Fc (5–10 μg/ml) (produced with a plasmid provided by D. L. Simmons, Imperial Cancer Research Fund, Oxford, U.K.) diluted in Tris-buffered saline pH 9.0 were coated onto polystyrene plates that were assembled as the lower wall in a parallel plate flow chamber and mounted on an inverted phase-contrast microscope (Diaphot TMD). Human naive and unfractionated T lymphocytes were isolated as described (29). The cells (at 1 × 106 cells/ml in Hanks’ balanced salt solution supplemented with 0.2% BSA) were incubated with SLC for 6 min at room temperature, perfused into the flow chamber, and incubated under static conditions for 6 min. Then flow was initiated and increased in 2-fold increments every 10 sec. The experiments were videotaped for analysis, and the number of cells remaining bound was determined at each interval by using National Institutes of Health’s image 1.6 analysis package. The same field of view was used for each experiment to ensure that the results reflected uniform site density and distribution of the immobilized protein. All adhesion experiments were performed a minimum of three times. Phorbol 12-myristate 13-acetate or Mn served as a control treatment to induce strong adhesion to the ICAM-1 substratum. For inhibition studies, the cells were pre incubated for 20 min with 10 μg/ml R3.1 (anti-αL, gift from S. Simon, Baylor University, Waco, TX) on ice.
RESULTS
Identification of SLC.
We searched the NCBI EST database by using human MCP-1 as a template and found several chemokine-like sequences. We then performed in situ hybridization on mouse tissues to identify those EST clones with expression patterns suggestive of novel activities. One mouse clone (I.M.A.G.E. Consortium clone ID 389013) hybridized strongly to secondary lymphoid tissues (as described below). Sequence analysis of this clone revealed a cDNA of 848 bp encoding a 133 amino acid protein (data not shown). Analysis of a closely related human EST (clone ID 503192) identified in the same screen revealed an 847 cDNA encoding a protein of 134 amino acids with 72% amino acid identity to the murine protein (data not shown). Both mouse and human predicted proteins contain a chemokine consensus pattern. Both proteins also contain a highly basic 34- (mouse) or 35- (human) amino acid C-terminal extension that includes two conserved cysteines. These proteins have recently been described by several reports in the literature (21, 23, 24). We refer to this chemokine by the name it was first given, SLC.
SLC Is Expressed in HEV, T Cell Areas, and Lymphatic Endothelium.
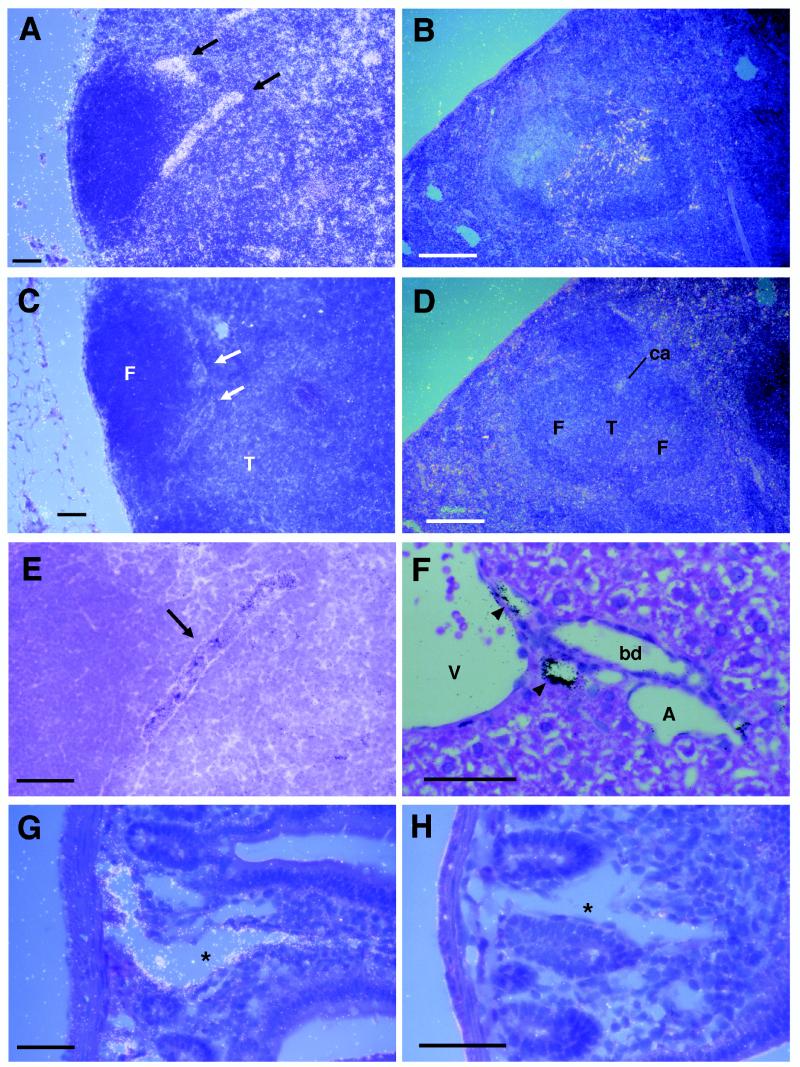
By in situ hybridization, we found SLC to be expressed at strikingly high levels in the HEV of lymph nodes (Fig. 1 A, E) and Peyer’s patches (data not shown). SLC was expressed at lower levels in the T-cell zones of lymph nodes (Fig. 1A), spleen (Fig. 1B), and Peyer’s patches (data not shown), but was undetectable in the B cell areas (follicles) of these organs. The distribution of signal in T cell zones was suggestive of expression by stromal cells rather than lymphocytes. SLC was also expressed in the endothelium of small vessels in multiple organs. These vessels were identified as lymphatics by their characteristic anatomic location in liver (Fig. 1F) and small intestine (Fig. 1G). SLC expression was not detected in brain (data not shown) or in arteries or veins. In control experiments, no hybridization was seen with an SLC sense probe (Fig. 1 C, D, H).
Figure 1.
Detection of SLC mRNA in mouse tissues by in situ hybridization. Sections were hybridized with antisense (A, B, E–G) or sense (C, D, H) 35S-labeled SLC riboprobe. (A) Dark field micrograph of SLC antisense probe hybridization in lymph node. Signal is seen as white dots. HEV are indicated by arrows. (B) SLC antisense probe hybridization in spleen. SLC sense probe does not hybridize to lymph node (C) or spleen (D). (E) High power view of HEV from A shown in bright field demonstrating typical morphology. Signal is seen as black dots. (F) Hybridization of SLC to the endothelium of small lymphatics (indicated by arrowheads) in liver. (G) Hybridization of SLC to the endothelial cells lining a central lactile (indicated by asterisks) in small intestine. (H) Lack of hybridization to SLC sense probe in small intestine. Identified structures are: F, follicle; T, T cell area; ca, central arteriole; A, artery; V, vein; bd, bile duct. [Bars = 100 μm (A–D) and 50 μm (E–H).]
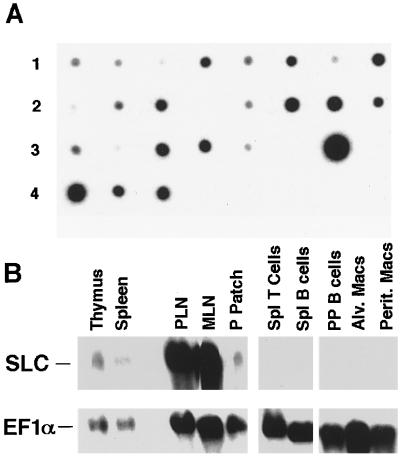
To corroborate the results of in situ hybridization, we performed Northern analysis on mRNA blots of human and mouse tissues. Hybridization of human SLC to a human multitissue dot blot revealed that this mRNA was most abundant in lymph nodes and appendix, with moderate amounts in spleen, small intestine, thyroid, pancreas, trachea, and salivary gland (Fig. 2A). Multiple tissues demonstrated low level hybridization. No signal was observed in any region of the brain (data not shown) or in peripheral blood lymphocytes. In mouse tissues, SLC was expressed most strongly in peripheral and mesenteric lymph nodes and at lower levels in Peyer’s patches, spleen, and thymus (Fig. 2B). Freshly isolated murine B cells, T cells, and macrophages were negative.
Figure 2.
Detection of SLC mRNA in tissues and purified cells by Northern analysis. (A) Autoradiograph of hSLC 32P-probe hybridization to a human multitissue dot blot. From left to right, tissues shown are: Row 1, heart, aorta, skeletal muscle, colon, bladder, uterus, prostate, stomach. Row 2, testis, ovary, pancreas, pituitary, adrenal, thyroid, salivary gland, breast. Row 3, kidney, liver, small intestine, spleen, thymus, peripheral leukocyte, lymph node, bone marrow. Row 4, appendix, lung, trachea, placenta. (B) Autoradiograph of mSLC 32P-probe hybridization to mouse northern blot. EF-1α hybridization is shown to indicate amounts of mRNA loaded in each lane.
SLC Stimulates Chemotaxis of Naive T Lymphocytes.
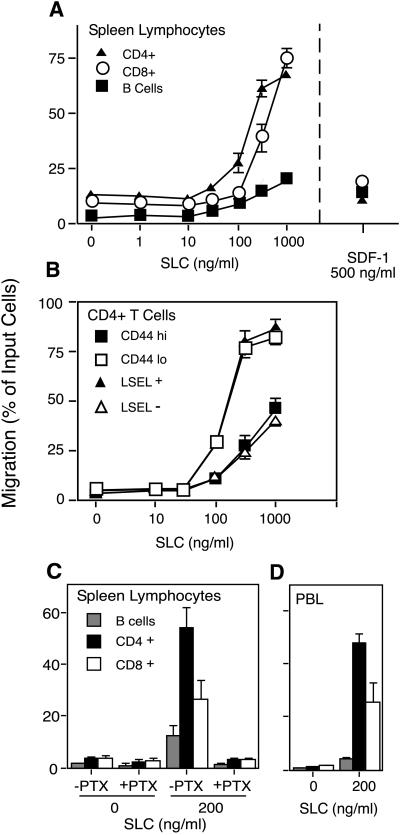
Because SLC is expressed in areas of T lymphocyte entry and accumulation, we investigated its chemotactic activity toward lymphocyte subsets. We found that mouse SLC was a highly efficacious chemoattractant for mouse splenic T lymphocytes, able to stimulate the migration of over 70% of the input T cells (Fig. 3A). At submaximal concentrations SLC showed greater activity toward CD4+ than CD8+ T cells. It exhibited less activity toward B cells. SLC attracted more T cells and had greater T cell specificity than SDF-1α, described previously as the most efficacious chemokine for resting lymphocytes (25). SLC showed a preferential activity toward those CD4+ T lymphocytes that expressed high levels of L-selectin and low levels of CD44 (Fig. 3B). In mice, this pattern of cell surface markers is indicative of naive lymphocytes (26, 27). The chemotactic response to SLC was inhibited by preincubation of lymphocytes with pertussis toxin (Fig. 3C), consistent with signaling through a Gαi-coupled receptor. To examine the effect of SLC on those cells that are normally recruited to secondary lymphoid organs, we measured the chemotactic response of mouse peripheral blood lymphocytes. SLC was a strong chemoattractant for these cells, with greatest activity toward CD4+ and CD8+ T cells (Fig. 3D).
Figure 3.
Chemotactic activity of SLC on mouse lymphocyte subtypes. Murine lymphocytes were subjected to chemotaxis through 5-μm pore Transwell filters. The number of input and migrating cells of each subtype were determined by immunostaining and flow cytometry. Results are expressed as the percentage of input cells of each subtype that migrated to the lower chamber. (A) Migration of B cells, CD4+ T cells, and CD8+ T cells to SLC. Peak migration of same cells to SDF-1α is shown for comparison. (B) Comparison of naive vs. memory cell migration in response to SLC. Naive CD4+ cells are defined as those expressing L-selectin (LSEL+) and having low levels of CD44 (CD44 lo). Memory cells are the converse (LSEL− and CD44 hi). (C) Inhibition of SLC-induced migration by pretreatment of cells for 2 hr with pertussis toxin (PTX). (D) Migration of mouse peripheral blood lymphocytes. Data points represent the mean ± SD for experiments performed in triplicate.
SLC Activates β2 Integrins on T Lymphocytes.
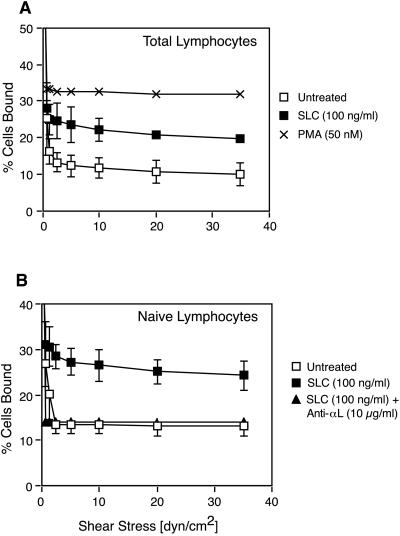
Because SLC is expressed in HEV and can stimulate the chemotaxis of naive lymphocytes, we predicted that it would also activate β2 integrins on T cells. To test this hypothesis, we measured the resistance of human lymphocytes to detachment by physiologic shear stress in a parallel plate flow chamber (19). SLC stimulated an increase in the percentage of T lymphocytes that remained attached to an ICAM-1 substrate as flow shear was increased (Fig. 4A). Naive T lymphocytes responded in a similar manner and this response could be blocked by an anti-αL antibody (Fig. 4B).
Figure 4.
SLC induces shear-resistant binding of total and naive T lymphocytes to immobilized ICAM-1. T Lymphocytes treated as indicated were allowed to attach to ICAM-1 for 6 min under static conditions in a parallel plate flow chamber. Flow was initiated and increased in 2-fold increments every 10 sec. Results are expressed as the percentage of input cells remaining bound at each flow rate. (A) Adhesion of SLC-treated, untreated and phorbol 12-myristate 13-acetate-stimulated T lymphocytes. (B) Effect of SLC on naive (CD45RA+) T lymphocytes compared with untreated cells and cells treated with SLC and an anti-αL antibody. Data points represent the mean ± SD for at least three separate experiments. Analysis by using a paired two-tailed Student’s t test showed that SLC induced statistically significant binding of both total T lymphocytes (P < 0.01) and naive T lymphocytes (P < 0.05) to ICAM-1.
DISCUSSION
In this study we demonstrate that SLC satisfies a number of important criteria for a chemokine that mediates lymphocyte homing to secondary lymphoid organs. First, SLC is expressed in the HEV of lymph nodes and Peyer’s patches, the major site of lymphocyte entry into these organs, and within T cell areas, the site where lymphocytes first accumulate, in spleen, lymph nodes, and Peyer’s patches. To our knowledge, this expression pattern is unique to SLC. Second, SLC is the most efficacious chemoattractant yet described for naive T lymphocytes, the cell type predominantly recruited to secondary lymphoid organs. Finally, SLC is the first chemokine demonstrated to stimulate the firm adhesion of lymphocytes to ICAM-1 under physiologic shear conditions, a step required for lymphocyte extravasation into lymph nodes and Peyer’s patches. Taken together, these findings strongly suggest that SLC functions as a lymphoid homing chemokine for lymphocytes.
Our findings provide new information about the role of SLC. We show that the SLC mRNA found in secondary lymphoid organs can be attributed to its expression in HEV and T cell areas. We find SLC to be expressed more broadly than reported (21, 23, 24) in nonlymphoid tissues and demonstrate that this is due to its expression on lymphatic endothelium. The role that SLC plays in lymphatic endothelium is unclear, but we hypothesize that it may recruit lymphocytes from tissues into draining lymphatics. The endothelial expression of SLC may provide an explanation for the conserved highly basic C-terminal extension found on this molecule. This motif may anchor SLC within vessels, perhaps by binding to specific anionic molecules on the endothelial glycocalyx.
The chemotactic activities of SLC that we observed toward T and B lymphocytes are most similar to those found by Hromas et al. (24). However, unlike that group, we found SLC to be most active toward naive rather than memory lymphocytes. This may reflect a species difference in the cells studied. Our results differ from those of Hedrick and Zlotnik, who found unstimulated mouse splenocytes to be unresponsive to SLC (23). This discrepancy could be due to their use of bacterially produced SLC that may differ in activity from protein made in eukaryotic cells.
Previous studies of the ability of chemokines to activate β2 integrins on lymphocytes have yielded contradictory results. Lloyd et al. (28) found that several chemokines can stimulate the adherence of peripheral blood lymphocytes to ICAM-1 coated slides. However, by using a parallel plate flow chamber, Carr et al. (19) failed to observe such an effect. The conditions we used to demonstrate the activation of β2 integrins by SLC are similar to those of the later study. The only other molecule that has been demonstrated to activate lymphocyte β2 integrin is GlyCAM-1, a secreted L-selectin ligand expressed by endothelial cells in HEV (29). It is possible that SLC and GlyCAM act cooperatively to stimulate the firm adhesion of lymphocytes to HEV. This interaction should be investigated in future studies. In addition, because SLC is expressed in the HEV of Peyer’s patches, it will be important to investigate the ability of SLC to activate α4β7 integrin, which is required for lymphocyte homing to this tissue.
One important issue that our results do not fully resolve is the subsets of lymphocytes acted on by SLC in vivo. While we have emphasized the effects of SLC on naive T lymphocytes, our findings suggest that SLC may also be capable of recruiting other lymphocyte types to secondary lymphoid organs. It has been suggested that only naive lymphocytes enter lymph nodes directly from the blood (30). However, more recent studies demonstrate that memory lymphocytes also follow this route, albeit less efficiently (31, 32). Thus, it is possible that the in vitro chemotactic effects of SLC on memory T cells are representative of an ability to stimulate the homing of these cells in vivo. In studies demonstrating memory lymphocyte homing, the ability of these cells to enter lymph nodes and Peyer’s patches was thought to be determined in part by their expression of L-selectin (32). It will be of interest to determine if the degree of memory lymphocyte homing correlates with the expression of an SLC receptor on these cells.
Consistent with published findings (24), we demonstrated that B lymphocytes are also attracted to SLC, though much less efficiently than T cells. Like T cells, B lymphocytes enter lymph nodes and Peyer’s patches by crossing HEV, and initially accumulate in T cell areas, where SLC is also expressed, before moving to follicles (33). Given these results, it is possible that SLC stimulates the entry of B cells into secondary lymphoid organs and that these cells are then attracted to follicles by a B cell specific chemokine such as the BLR1 ligand (34). However, in view of the lower responsiveness of B cells to SLC relative to that of T cells, we find it equally plausible that another chemokine is responsible for the homing of B cells to secondary lymphoid organs.
A second unresolved issue is the cell type that expresses SLC in the T cell areas of spleen, lymph nodes, and Peyer’s patches. SLC mRNA is not detected in lymphocytes by Northern hybridization and Hedrick found that SLC was not represented in cDNA libraries derived from lymphocytes or dendritic cells (23), suggesting that these cells types are unlikely to be a source. Consistent with these later results, we were unable to detect SLC message in purified interdigitating dendritic cells by in situ hybridization (data not shown). The expression of SLC by high endothelial cells and lymphatic endothelial cells suggests that endothelial cells within T cell areas may also be a source. However, expression by a population of macrophages or fibroblastic reticular cells is also possible.
In conclusion, our findings demonstrate that SLC has the characteristics of a lymphoid organ homing chemokine. In accord with current multistep models of leukocyte trafficking (3, 4), we would predict that SLC, localized at high local concentrations on HEV, interacts with a G protein coupled receptor on rolling lymphocytes. Receptor occupancy, perhaps in conjunction with L-selectin mediated signals (29), stimulates an increase in β2 integrin-mediated adhesion to counterreceptors such as ICAM-1. Firmly attached lymphocytes may then follow a chemotactic or haplotactic (35) gradient into the T cell zone. The results presented here, when considered with our recent identification of a chemokine that stimulates the migration of B cells into lymphoid follicles (M.D.G., V. N. Ngo, K. M. Ansel, E. H. Eckland, J.G.C., and L.T.W.; unpublished manuscript), support the emerging view that chemokine are major cues that control the migration of lymphocytes into and within lymphoid organs.
Acknowledgments
We thank Ms. Linda Prentice and Ms. Dale Milfay for their excellent technical assistance. This work was supported by an unrestricted award from the Howard Hughes Medical Institute (M.D.G. and L.T.W.) and National Institutes of Health Grant R37GM23547 (S.D.R.). K.T. was supported by Deutsche Forschungsgemeinschaft Award Ta209/1-1.
ABBREVIATIONS
- HEV
high endothelial venule
- SLC
secondary lymphoid-tissue chemokine
- EST
expressed sequence tag
- ICAM
intercellular adhesion molecule
References
- 1.Girard J-P, Springer T A. Immunol Today. 1995;16:449–457. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 2.van Ewijk W, Nieuwenhuis P. Experientia. 1985;41:199–208. doi: 10.1007/BF02002614. [DOI] [PubMed] [Google Scholar]
- 3.Springer T A. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 4.Butcher E C, Picker L J. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 5.Butcher E C. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 6.Rosen S D, Bertozzi C R. Curr Opin Cell Biol. 1994;6:663–673. doi: 10.1016/0955-0674(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 7.Camp R, Scheynius A, Johansson C, Puré E. J Exp Med. 1993;178:497–507. doi: 10.1084/jem.178.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamann A, Jablonski-Westrich D, Duijvestijn A, Butcher E, Baisch H, Harder R, Thiele H. J Immunol. 1988;140:693–699. [PubMed] [Google Scholar]
- 9.Hu M, Crowe D, Weissman I, Holzmann B. Proc Natl Acad Sci USA. 1992;89:8254–8258. doi: 10.1073/pnas.89.17.8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berlin C, Berg E L, Briskin M J, Andrew D P, Kilshaw P J, Holzmann B, Weissman I L, Hamann A, Butcher E C. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 11.Oppenheim J J, Zachariae C O C, Mukaida N, Matsushima K. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 12.Baggiolini M, Dewald B, Moser B. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 13.Vaddi K, Newton R C. J Immunol. 1994;153:4721–4732. [PubMed] [Google Scholar]
- 14.Tanaka Y, Adams D H, Hubscher S, Hirano H, Siebenlist U, Shaw S. Nature (London) 1993;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- 15.Huber A, Kunkel S, Todd R D, Weiss S. Science. 1991;254:99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- 16.Bargatze R F, Butcher E C. J Exp Med. 1993;178:367–372. doi: 10.1084/jem.178.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spangrude G, Braaten B, Daynes R. J Immunol. 1984;132:354–362. [PubMed] [Google Scholar]
- 18.Cyster J G, Goodnow C C. J Exp Med. 1995;182:581–586. doi: 10.1084/jem.182.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr M W, Alon R, Springer T A. Immunity. 1996;4:179–187. doi: 10.1016/s1074-7613(00)80682-2. [DOI] [PubMed] [Google Scholar]
- 20.Adema G J, Hartgers F, Verstraten R, de Vries E, Marland G, Menon S, Foster J, Xu Y, Nooyen P, McClanahan T, et al. Nature (London) 1997;387:713–717. doi: 10.1038/42716. [DOI] [PubMed] [Google Scholar]
- 21.Nagira M, Imai T, Hieshima K, Kusuda J, Ridanpaa M, Takagi S, Nishimura M, Kakizaki M, Nomiyama H, Yoshie O. J Biol Chem. 1997;272:19518–19524. doi: 10.1074/jbc.272.31.19518. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida R, Imai T, Hieshima K, Kusuda J, Baba M, Kitaura M, Nishimura M, Kakizaki M, Nomiyama H, Yoshie O. J Biol Chem. 1997;272:13803–13809. doi: 10.1074/jbc.272.21.13803. [DOI] [PubMed] [Google Scholar]
- 23.Hedrick J, Zlotnik A. J Immunol. 1997;159:1589–1593. [PubMed] [Google Scholar]
- 24.Hromas R, Kim C, Klemsz M, Krathwohl M, Fife K, Cooper S, Schnizlein-Bick C, Broxmeyer H. J Immunol. 1997;159:2554–2558. [PubMed] [Google Scholar]
- 25.Bleul C C, Fuhlbrigge R C, Casasnovas J M, Aiuti A, Springer T A. J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Budd R, Cerottini J, Horvath C, Bron C, Pedrazzini T, Howe R, MacDonald H. J Immunol. 1987;138:3120–3129. [PubMed] [Google Scholar]
- 27.Jung T, Gallatin W, Weissman I, Dailey M. J Immunol. 1988;141:4110–4117. [PubMed] [Google Scholar]
- 28.Lloyd A, Oppenheim J, Kelvin D, Taub D. J Immunol. 1996;156:932–938. [PubMed] [Google Scholar]
- 29.Hwang S T, Singer M S, Giblin P A, Yednock T A, Bacon K B, Simon S I, Rosen S D. J Exp Med. 1996;184:1343–1348. doi: 10.1084/jem.184.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackay C R. Adv Immunol. 1993;53:217–265. doi: 10.1016/s0065-2776(08)60501-5. [DOI] [PubMed] [Google Scholar]
- 31.Westermann J, Pabst R. Immunol Today. 1996;17:278–282. doi: 10.1016/0167-5699(96)80545-7. [DOI] [PubMed] [Google Scholar]
- 32.Williams M, Butcher E. J Immunol. 1997;159:1746–1752. [PubMed] [Google Scholar]
- 33.Goodnow C C, Cyster J G. Curr Biol. 1997;7:R219–R222. doi: 10.1016/s0960-9822(06)00105-9. [DOI] [PubMed] [Google Scholar]
- 34.Forster R, Mattis A E, Kremmer E, Wolf E, Brem G, Lipp M. Cell. 1996;87:1–20. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 35.Rot A. Immunol Today. 1992;13:291–294. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- 36.Pearson W, Lipman D. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lennon G, Auffray C, Polymeropoulos M, Soares M B. Genomics. 1996;33:151–152. doi: 10.1006/geno.1996.0177. [DOI] [PubMed] [Google Scholar]
- 38.Adams M D, Kerlavage A R, Fleischmann R D, Fuldner R A, Bult C J, Lee N H, Kirkness E F, Weinstock K G, Gocayne J D, White O, et al. Nature (London) 1995;377:3–174. [PubMed] [Google Scholar]