Abstract
Human 15-lipoxygenase (h15-LO) is present on chromosome 17p13.3 in close proximity to the tumor-suppressor gene, p53. 15-LO is implicated in antiinflammation, membrane remodeling, and cancer development/metastasis. The murine BALB/c embryo fibroblast cell line, (10)1val, expresses p53 in mutant (mt) conformation when grown at 39°C and in wild-type conformation when grown at 32°C. Transfection of h15-LO promoter constructs (driving luciferase reporter) into (10)1val cells and into p53-deficient (10)1 cells resulted in a marked increase in h15-LO promoter activity in (10)1val cells at 39°C, but not at 32°C, or as compared with (10)1 cells. Transfection of h15-LO promoter deletion constructs, however, resulted in total loss of activity in both cell types at 32°C and 39°C. Cotransfection of (10)1 cells with h15-LO promoter (driving luciferase reporter) along with increasing levels of a mt p53 expression vector demonstrated dose-dependent capacity of mt p53 to induce 15-LO promoter activity. No effect was observed with wild-type p53. In contrast to h15-LO promoter activity, (10)1val cells had significantly lower levels of endogenous (murine) 12/15-LO (mouse analog of h15-LO) mRNA and protein when grown at 39°C compared with cells grown at 32°C. Our data support the hypothesis that loss of a tumor-suppressor gene (p53), or “gain-of-function activities” resulting from the expression of its mutant forms, regulates 15-LO promoter activity in man and in mouse, albeit in directionally opposite manners. The studies define a direct link between 15-LO activity and an established tumor-suppressor gene located in close chromosomal proximity.
Lipoxygenases are lipid-peroxidating enzymes that are implicated in the pathogenesis of a variety of inflammatory disorders, as well as membrane remodeling, carcinogenesis, and atheroma formation (1–4). Formation of 15-(S)-hydroxyeicosatetraenoic acid [15-(S)-HETE] and lipoxin A4 in human leukocytes, mediated by human 15-lipoxygenase (15-LO)-dependent catalysis of arachidonic acid, likely represents a component of endogenous antiinflammatory influences that ultimately regulate the extent and severity of inflammatory reactions. The close chromosomal localization of 15-LO to that reported previously for 12-LO (5, 6) is consistent with a common evolutionary origin. In fact, by DNA homology studies in mice, rats, and other species, the two enzymes apparently “merge” into a single rodent protein (12/15-LO), which performs both 12 and 15 lipoxygenations (2–4). It should be recognized, however, that h15-LO may not be a strict functional equivalent of murine 12/15-LO. The present findings indeed support a functional divergence of the two enzymes at the level of transcriptional regulation.
During the past decade, the tumor-suppressor protein p53 became one of the most studied molecules in cancer research (7). A high frequency of mutations of the p53 gene is found in most human cancers (8), and, in those tumors in which p53 itself is not mutated, other factors may modulate its function to render it inactive. p53 is a nuclear phosphoprotein whose inactivation, either through mutation, selective interaction with cellular MDM2 protein, viral oncogene products, or alteration in subcellular localization, is correlated strongly with human cancer (9–11). The p53 protein possesses tetramerization, DNA-binding, and trans-activation functions residing in separate polypeptide domains (12, 13). Depending on the specific cell type, the expression of wild-type (wt) p53 has been shown to result in G1 checkpoint control of cell growth or apoptotic cell death (14–16).
Convincing evidence suggests that p53-mediated cell cycle arrest at the G1 checkpoint is induced by various types of DNA damage (17–20). Wt p53 can positively regulate expression of a number of downstream effector genes, including the GADD45 (18), p21Waf/Cip1 (21), cyclin G (22), mdm-2 (23, 24), and mouse muscle creatine kinase genes (25). In addition, wt p53 negatively regulates a variety of genes that lack a p53 consensus-binding site, including c-fos (26, 27), MDR1 (28), heat-shock protein 70 (29), interleukin 6 (30), proliferating cell nuclear antigen (31, 32), O6-methylguanine-DNA methyltransferase (33), as well as other viral and cellular promoters (32). It has been suggested that transcriptional repression by p53 results from its direct interaction with transcription factors such as TATA-binding protein (34–36), Sp1 (37), and CCAAT-binding factor (CBF) (29). Taken together, these observations strongly imply that p53 acts directly with the transcription machinery to modulate transcription. The loss or inactivation of wt p53 increases genomic instability (38, 39) and susceptibility to malignant transformation (40, 41). Defects in the cell-cycle checkpoint thus may contribute to the genomic instability of cancer cells in which chromosomal rearrangements, translocations, and gene amplification occur with increased frequency (42).
In parallel, there has been strong evidence providing a link between 15-LO, linoleic acid metabolism, and the development or progression of prostate cancer and colorectal cancer (43, 44). Studies by Hussey and Tisdale (43) support the concept that some tumors are dependent on the lipoxygenase metabolites of linoleic acid (LA) and arachidonic acid (AA) for their continued growth and that interference with lipoxygenase activities produces specific growth inhibition. Tang et al. (45) suggest that AA and other polyunsaturated fatty acids and/or their metabolites may enhance tumor growth not only by promoting cell proliferation, but also by suppressing apoptosis. It also has been demonstrated that protein kinase C and phosphatases are involved in 12(S)-HETE-induced tumor cell cytoskeletal reorganization and actin microfilament content of adherent B16 amelanotic melanoma (B16a) cells (46, 47). Hussey and Tisdale (48) have shown that lipoxygenase inhibitors inhibit lung cancer growth and prevent lung carcinogenesis and that the inhibitory effects of these agents on cell growth result from an imbalance of metabolism of arachidonic acid between the 5-, 12-, and 15-lipoxygenase pathways. Because the products of 12- and 15-LO are implicated in cancer formation or metastasis, one of the fundamental properties of p53 tumor-suppressor activity may reside in its regulation of the expression of these two genes, i.e., 12/15-LO and 15-LO.
Many aspects of the biological function and activity of lipoxygenases are fairly well characterized; however, the mechanisms governing the regulation of their encoding genes remain to be elucidated fully. Effects of p53 on 12- and 15-lipoxygenases have, until now, been unexplored. In the present study, we show that expression of mutant (mt p53) up-regulates human 15-lipoxygenase promoter activity, whereas it markedly inhibits mouse 12/15-lipoxygenase expression. Thus, mt p53 and not wt p53 exhibits a gain-of-function activity (regulation of 15- and 12/15-LO) and may contribute to carcinogenesis not only by absence of tumor-suppressor activities, but also by the emergence of species-specific, dramatic alterations in 15- and 12/15-LO gene expressions in humans and mice, respectively.
MATERIALS AND METHODS
Cell culture medium and supplements were purchased from GIBCO/BRL. Restriction enzymes and other related reagents were purchased from Promega or GIBCO/BRL. 32P-labeled nucleoside triphosphates were purchased from DuPont/NEN. Nitrocellulose filters were bought from Amersham. Antibodies to wt and mt p53 proteins [anti-p53 (Ab-7)] were purchased from Calbiochem. Luminol/enhancer solutions were obtained from Amersham Pharmacia. Unless otherwise stated, all other chemicals were purchased from Sigma.
Plasmids and Cell Culture.
The human p53 expression plasmids and the empty vector used in this study have been described previously (9). All of the p53 constitutive expression constructs were produced with a cytomegalovirus (CMV) promoter–enhancer expression vector. Briefly, a wt p53 cDNA was inserted into pCMV-Neo-Bam vector to express p53 (originally named pC53-SN3). Other vector-expressing mt human p53 was constructed similarly. p53–175 is a mt human p53 cDNA expression plasmid containing a substitution of histidine for arginine at amino acid 175.
p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts (40), contains large deletions in both p53 alleles, and, consequently, is deficient in the p53 protein. The (10)1val cell line was developed from (10)1 cells by transfection with an expression vector, producing a temperature-sensitive p53 protein (24, 40). At 32°C, the p53 protein is predominantly in the wt conformation, whereas at 39°C, most of the p53 protein takes on a mt form. Cells were cultured in DMEM containing 10% FBS/2 mM glutamine/100 units/ml of penicillin/100 μg/ml of streptomycin/25 μg/ml of fungizone in 5% CO2 at 37°C.
Preparation of Protein Extracts.
The (10)1 and (10)1val cells were plated 24 h before the duplicate plates of cells were shifted to either 39 or 32°C. After 24 h at the appropriate temperature, cells were trypsinized from the plate, washed once with ice-cold PBS containing 1 mM PMSF, and centrifuged at 1,000 × g for 4 min. The cell pellet then was processed for lysate protein extraction. In all buffers, protease inhibitors were added just before use: PMSF and benzamidine at 1 mM each, aprotinin and leupeptin at 10 μg/ml, and pepstatin A at 1 μg/ml. Protein concentrations were determined by the Bio-Rad protein assay. The proteins were stored in aliquots at −70°C.
SDS/PAGE and Western Blotting.
For detection of 12/15-lipoxygenase (12/15-LO) protein, a 25-μg sample of cell lysate protein (2 × 106 cells) extract was mixed with loading buffer, boiled for 5 min, and then loaded onto a 10% polyacrylamide gel containing 0.1% SDS. The gel was run at 200 V for 40 min. The separated proteins were transferred onto a nitrocellulose membrane by electroblotting. Equivalent loading was checked by Ponceau S staining of the blot. After being blocked with 5% nonfat dry milk in PBS, the nitrocellulose membrane was incubated with polyclonal 12-lipoxygenase (LX12A) antiserum (Oxford Biomedical Research, Oxford, MI) for 1 h at room temperature. After incubation with donkey anti-sheep peroxidase second antibody (1:10,000 dilution), the 12/15-LO protein was visualized by using the Luminol/Enhancer (enhanced chemiluminescence, ECL) solutions as described by the manufacturer.
For detection of p53 protein, 25 μg of cell lysate protein was mixed with loading buffer, boiled for 5 min, loaded onto a 10% polyacrylamide gel, electrophoresed, and transferred to a nitrocellulose membrane as described above. The membrane was blocked overnight with 5% nonfat dry milk in PBS and incubated for 1 h at room temperature with the p53 antibody (1:1,500 dilution). After incubation with rabbit anti-sheep peroxidase second antibody (1:5,000 dilution), the p53 protein was visualized by using the Luminol/Enhancer as described above.
Isolation and Analysis of mRNAs.
The (10)1 and (10)1val cells were plated in 10-cm-diameter plates for 24 h at 37°C before being shifted to either 39 or 32°C for an additional 24 h. The cells then were harvested, and RNA was extracted for Northern blot analysis as described previously (49). Briefly, 25 μg of total RNA was electrophoresed on a 1% agarose/6% formaldehyde gel and transferred to a Duralon-UV membrane, which was then UV cross-linked and subjected to prehybridization and then hybridization with the 12/15-LO cDNA probe. After hybridization, the membrane was washed and exposed to x-ray film for autoradiography. The same membrane then was stripped and rehybridized with α-actin probe as an internal standard. The DNA probe used to analyze 12/15-LO mRNA was a 106-bp DNA fragment from the coding region of a rat 12/15-LO gene (4). A 1.5-kb full-length cDNA clone of rat cytoplasmic α-actin (Stratagene) was used as the α-actin probe.
Construction of Plasmids for Transient Expression.
The 5′ flank sequence of the human 15-LO gene was PCR-amplified by using different primers. The products were subcloned into a luciferase reporter vector, pGL2-basic (Promega). The integrity of all constructs was determined by restriction enzyme site mapping, and all constructs produced by PCR were sequenced by using fluorescent methodology.
To identify DNA sequence regulating 15-LO gene expression, the region from −628 to −23 bp (+1 is adenine in the ATG start codon) was amplified by PCR from the 3-kb fragment cloned in LOPB-5, using the adapter as sense and an antisense primer. The sequence of the primers were as follows: adapter as sense primer, 5′-TTAAAGGTACCGCAGACAACAGGGAGGC-3′ (KF-1); antisense primer, 5′-TTTAACCCAAGCTTCTGGTGGAGAAGAAGGGTGG-3′ (HR-1). The 605-bp PCR product was purified and restricted with KpnI and HindIII and cloned into a plasmid pGL2 containing the luciferase reporter gene (Promega). The resulting plasmid LOPB5 and the other plasmids as described below then were transiently transfected into the (10)1 and (10)1val cells. The luciferase activity assay demonstrated that the LOPB5 clearly had promoter activity in the cells tested. To determine further the site of the minimal 15-LO promoter region, a series of deletion mutants was constructed by using the previously described procedure. Therefore, individual fragments of various sizes encompassing the promoter region were subcloned in the plasmid pGL2. Three antisense primers, 5′-TTTAACCCAAGCTTTACAGCAGGCAGGCGAGG-3′ (HR-2) and 5′-TTAACCCAAGCTTCAAGGGGCAGATTCA-3′ (HR-3), and a sense primer, 5′-TTAAAGGTACCGTGGTACACACGT GC-3′ (KF-2), were used to generate PCR-amplified fragments of various sizes.
A 318-bp fragment, namely LOKB1 (−628 to −310 nt) with KF-1 and HR-2, a 338-bp fragment, LOKB2 (−361 to −23 nt) with KF-2 and HR-1, and a 196-bp fragment, LOKB3 (−361 to −165 nt) with KF-2 and HR-3, were generated and cloned in the reporter plasmid as described (49).
Transient Transfections and Dual-Luciferase and Protein Assays.
Cells were plated at 40% confluency in 12-well plates. After 24 h, the plasmid DNA (1 μg) was transfected into the cells by FuGENE 6 Transfection reagent (Boehringer Mannheim). Luciferase plasmids were cotransfected with coreporter pRL-SV40 vector promoter upstream of Rluc (Promega) into (10)1 and (10)1val cells to monitor transfection efficiency. The cells were grown for 24 h before being harvested for assays. The cell extracts were prepared with the Passive lysis buffer (Promega). The cell lysate was analyzed for protein concentration (Bio-Rad) as well as for dual-luciferase reporter assay by using a luminometer with autoinjector (Turner design), as described by the manufacturer (Promega). The experiments were performed in duplicate or triplicate. Simultaneously individual expression of pRL-SV40 was checked for inhibition or increased expression by wt as well as mt p53.
RESULTS AND DISCUSSION
Transcriptional Activity of the Human 15-LO Promoter in (10)1 Cells.
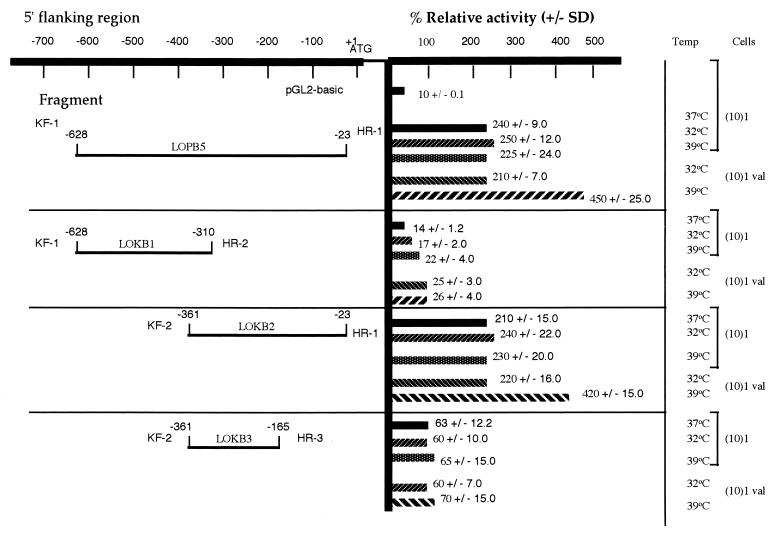
Human 15-LO is not normally expressed in murine fibroblasts. Hence, we studied the effects of p53 by using cloned human promoter as described in Materials and Methods. Toward this end, human 15-LO promoter–luciferase reporter plasmids were constructed with varying lengths of 5′ promoter sequences (Fig. 1). (10)1 cells were used in the transient transfection assays because of their negative p53 background, which is ideal for studying the role of p53 in the transcriptional regulation of exogenously transfected gene promoters (19, 30). As shown in Fig. 1, the reporter construct (LOPB5) of 600 bp containing human 15-LO promoter sequences gave maximal transcriptional activity in (10)1val cells grown at 39°C and none at 32°C as compared with (10)1 cells transfected and grown under similar conditions. Stepwise deletion of 5′ sequences from the promoter resulted in a concomitant decrease in the luciferase activity in both (10)1 and (10)1val cells grown at 32o and 39°C, respectively, except by the LOKB2 construct.
Figure 1.
Schematic linear map of human 15-LO promoter–luciferase plasmids and their transcription activity in (10)1 and (10)1val cells. Plasmids containing various lengths of human 15-LO promoter 5′ of the luciferase gene were constructed as described in Materials and Methods. The sequence numbers in the map correspond with those published by Kelavkar et al. (49), with the major transcription start site set to +1. Plasmid DNA (1 μg) was transfected into (10)1 cells by FuGENE 6 Transfection reagent. The cells were grown in appropriate medium containing 10% FBS at 37, 32, and 39°C, respectively, for 24 h, depending on individual experiments, before being harvested for determination of luciferase activity. Each experiment was repeated at least three times with duplicate samples. The values shown on the right are the averages ±SD of the relative promoter activities.
Human 15-LO Promoter Activity in (10)1 and (10)1val Cells.
Expression of wt p53 had no effect on the expression of murine 12/15-LO mRNA and protein in (10)1val cells grown at 32°C whereas, surprisingly, at 39°C there was decreased expression (Figs. 5 and 6). To test whether similar effects are seen in p53 interaction with the human 15-LO promoter activity, the promoter–luciferase reporters, namely, LOPB5, LOKB1, LOKB2, and LOKB3, were transiently transfected, in duplicate, into (10)1 and (10)1val cells at 37°C and then shifted to 32 or 39°C for 20 h. Transfection of 15-LO basal promoter and deletion constructs lacking regions essential for promoter activity into p53-deficient (10)1 and (10)1val cells expressing mt or wt p53, depending on temperatures grown, indicates an increase in activity in (10)1val cells at 39°C with no effect at 32°C in the basal promoter, compared with (10)1 cells transfected similarly. However, in the deletion constructs there was a loss in activity in cells at both 32°C and 39°C, respectively, except by the LOKB2 construct (Fig. 1). These data were substantiated further by using (10)1 cells cotransfected with wt p53 and 15-LO promoter constructs and grown at 32°C and, simultaneously, mt 53 and 15-LO promoter constructs grown at 39°C (Fig. 2). These results together demonstrate that expression of p53 in the mt conformation decreased murine 12/15-LO transcriptional activity but increased the human 15-LO promoter luciferase activity. These differences reflect reciprocal regulation of these genes by p53 in mice vs. humans, highlighting the extreme care that must be exercised in interpreting mice data with respect to the biology of tumor-suppressor functions.
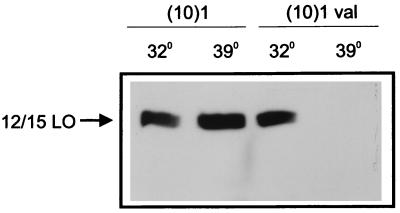
Figure 5.
Expression of mt p53 reduces 12/15-LO protein. (10)1 and (10)1val cells were plated and incubated at 37°C for 24 h before being shifted to either 39 or 32°C for another 24 h. Nuclear and cytoplasmic proteins were extracted for Western blotting as described in Materials and Methods. The 75-kDa protein band reactive with 12-LO antiserum is indicated. (10)1val cells have predominantly wt p53 at 32°C but have mutant p53 at 39°C. (10)1 cells contain no p53 protein.
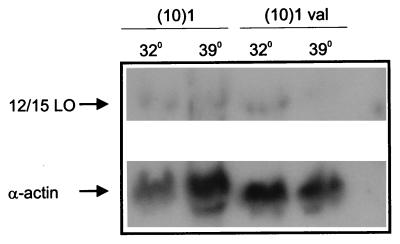
Figure 6.
Expression of mt p53 decreases the steady-state level of 12/15-LO mRNA. (10)1 and (10)1val cells were plated and incubated at 37°C for 24 h before being shifted to either 39 or 32°C for another 24 h. Total RNA was extracted for Northern blotting as described in Materials and Methods. The blot first was probed with a 32P-radiolabeled insert of the mouse 12/15-LO gene. After being stripped, the membrane was reprobed with mouse α-actin.
Figure 2.
Effects of wt or mt p53 on the 15-LO promoter in (10)1 cells. (10)1 cells were cotransfected with LOPB5 (1.0 μg) along with wt or mt p53 expression plasmid DNA (0.1 μg). The amount of DNA in each transfection was normalized with empty vector. The cells were grown in appropriate medium containing 10% FBS for 24 h before being harvested for determination of luciferase activity. Each experiment was repeated twice with triplicate samples. The values are expressed as a percentage of the luciferase activity in lysates from cells cotransfected with the control CMV-Neo-Bam vector only and are the means (±SD) of activities from triplicate samples in a representative experiment.
mt p53 Enhances Promoter Activity of Human 15-LO.
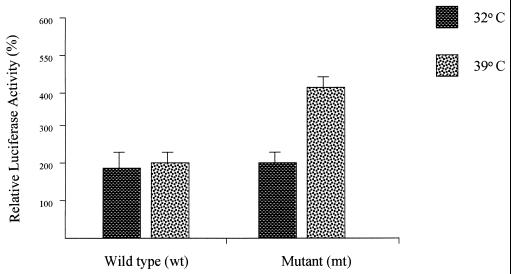
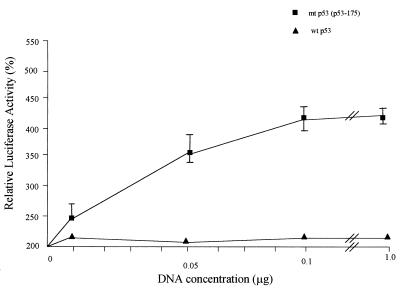
To study the effects and possible interactions, if any, of p53 expression with the human 15-LO gene promoter, (10)1 cells were transfected with LOPB5 along with various amounts of expression plasmids encoding wt or mt p53 (Fig. 3). Transfection with the wt p53 expression plasmid did not show any effect on the 15-LO promoter activity whereas that with the mt p53 expression plasmid at levels as low as 10 ng caused a significant increase of activity (4- to 5-fold compared with wt p53). Increasing amounts of mt p53 plasmid used in the transfection resulted in increased 15-LO promoter activity (Fig. 3). However, greater amounts of p53 plasmid did not increase the induction proportionally, indicating a saturable mechanism for this p53 function. Also, transfection with low or as high as 1.0-μg levels of wt p53 expression plasmid had no significant effect on the 15-LO promoter activity.
Figure 3.
Effects of different amounts of p53 on human 15-LO promoter activity in (10)1 cells. (10)1 cells were cotransfected with LOPB5 (1.0 μg) and various amounts of wt or mt p53 expression plasmid. The DNA for each transfection was brought up to 2.0 μg with the CMV-Neo-Bam control vector. The cells were grown with 10% FBS in medium at appropriate temperatures for 24 h and harvested for luciferase assay. Each experiment was repeated three times with duplicate samples. Values are expressed as percentages of the control activity (0%) in lysates from cells cotransfected with LOPB5 and the CMV-Neo-Bam control vector and are means ± SD (bars) of luciferase activities from duplicate samples in a representative experiment.
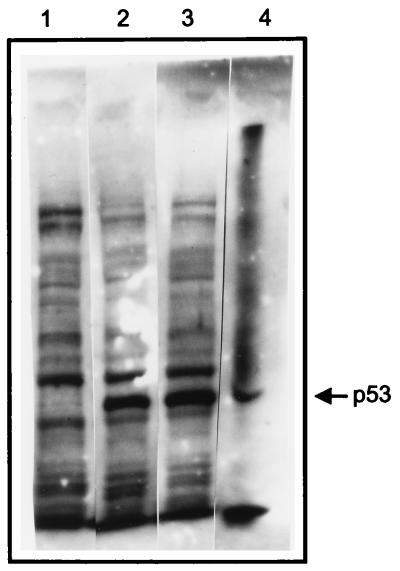
Western blot analyses of protein from transfected cell lysates were performed to confirm that wt and mt p53 proteins were produced in the cells transfected with the p53 expression vectors. With transfection of 1.0 μg of the p53 construct DNA, similar levels of wt and mt p53 were detected (Fig. 4). These results confirm that the significant effect on 15-LO promoter activity by mt p53 is because of mt p53 expression in the transfected cells and is unaffected by wt p53.
Figure 4.
Expression of wt and mt p53 in transfected (10)1 cells. (10)1 cells were transfected with wt or mt p53 expression plasmid DNA (1.0 μg) without any 15-LO promoter vector. The cells were grown and lysates were prepared as described in Fig. 3. Western blotting was conducted as described in Materials and Methods. Lanes: 1, (10)1; 2, wt p53; 3, p53–175 (mt p53); and 4, Escherichia coli-expressed p53 protein (standard).
Expression of mt p53 Reduces Murine 12/15-LO protein.
To study the effect of p53 expression on endogenous 12/15-LO protein, we used the murine fibroblast (10)1 cell line, which is negative for p53 protein, and the derivative (10)1val cell line, which expresses a temperature-sensitive p53 protein. When the (10)1val cells are grown at 32°C, the protein is predominantly in the wt conformation, whereas at 39°C, most of the p53 protein is in the mutant conformation. Western blot analysis showed that (10)1val cells incubated at 39°C for 24 h had a dramatically decreased level of 12/15-LO protein compared with the cells incubated at 32°C (Fig. 5). The decrease in 12/15-LO protein is a consequence of mt p53 and not temperature effects, because shifting (10)1 cells to 32°C for the same time period had a minimal effect on the expression of 12/15-LO protein.
Expression of mt p53 Decreases the Steady-State Level of Murine 12/15-LO.
The decreased amount of 12/15-LO protein in cells expressing mt p53 could result from either pre- or posttranslational events. With the same cell lines and temperatures as described for Fig. 5, the effect of p53 expression on endogenous 12/15-LO mRNAs was determined by Northern blot analysis. As shown in Fig. 6, the steady-state level of 12/15-LO was decreased markedly by mt p53 expression, as reflected by a sharp reduction of the 12/15-LO mRNA in (10)1val cells when they are incubated at 39°C for 24 h rather than at 32°C. Under the same conditions, the temperature had no effect on the level of 12/15-LO mRNA in (10)1 cells. When reprobed with α-actin cDNA as an internal loading control, the same membrane showed similar levels of α-actin mRNA at either temperature in both cell lines, indicating that mt p53 selectively inhibits 12/15-LO gene expression. Thus, mt p53 expression in (10)1val cells at permissive temperatures effectively decreases 12/15-LO mRNA levels, thus reducing 12/15-LO protein at a translational level.
Mutant p53 has been shown previously to bind abnormally to DNA in vitro (50) and only recently to have specific binding (51) by different classes of human p53 mutants (52–54). Mutant p53 proteins also have been shown to stimulate the expression of some genes, such as the multidrug resistance (MDR) gene (28), and bind to MAR/SAR DNA elements (51). Investigations therefore are underway to understand the direct interaction between p53 and probable suppressors or binding proteins that stimulate the expression of h15-LO and repress the activity of murine 12/15-LO.
The present studies suggest that mt p53 stimulation may be a form of “squelching,” whereby one or more factors inhibitory or regulatory for the transcription of LOs are regulated secondarily. Consistent with this idea, similar results have been obtained by others (55–58). Our data with 15-LO and p53 studies propose an interesting prediction: tumors with mutant missense p53 protein may well be more aggressive or have a poorer prognosis than tumors with no p53 proteins (deletions impart only a “loss of function” mutation). Thus, it may be that a missense p53 mutation contributing a “gain of new function” is worse than no p53 gene product at all. Previous studies demonstrating that lipoxins inhibit human natural killer cell cytotoxicity (1), thereby potentially hampering tumor surveillance, suggest a synergizing (or even mediating?) effect for these 15-LO products in enhancing the tumor-promoting action of mt p53. 15-LO expression then would be the first demonstration of a “gain-of-function” mutation imparted by p53 mutagenesis, which would promote carcinogenesis. Additionally, activation of 15-LO may lead to the generation of non-arachidonate-derived oxidation products such as 13-HODE (linoleic acid as substrate), which have been implicated in tumor metastasis (59). Further, non-eicosanoid-related activities of 15-LO, such as peroxidation of membrane lipids, also may contribute to alterations in cellular functions (60) arising from its de novo induction in cells containing mutant p53.
Identification of targets such as 15-LO that seem to be regulated by mutant p53, secondarily acting as mutator genes, is critical to our understanding of human tumor biology and to the development of cancer therapies.
Acknowledgments
We thank G. Zambetti, St. Jude’s Children’s Hospital, Tennessee, for valuable advice and useful reagents. This work was supported in part by National Institutes of Health Grant 2RO1DK43883 (to K.F.B).
ABBREVIATIONS
- 15-LO
15-lipoxygenase
- 12/15-LO
12/15-lipoxygenase
- CMV
cytomegalovirus
- wt
wild type
- mt
mutant
References
- 1.Samuelsson B, Dahlen S E, Lindgren J A, Rouzer C A, Serhan C N. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 2.Badr K F, DeBoer D K, Schwartzberg M, Serhan C N. Proc Natl Acad Sci USA. 1989;86:3438–3442. doi: 10.1073/pnas.86.9.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badr K F. Curr Opin Nephrol Hypertens. 1997;6:111–118. [PubMed] [Google Scholar]
- 4.Katoh T, Lakkis F G, Makita N, Badr K F. Kidney Int. 1994;46:341–349. doi: 10.1038/ki.1994.280. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimoto T, Arakawa T, Hada T, Yamamoto S, Takahashi E. J Biol Chem. 1992;267:24805–24809. [PubMed] [Google Scholar]
- 6.Funk C, Funk L, FitzGerald G, Samuelsson B. Proc Natl Acad Sci USA. 1992;89:3962–3966. doi: 10.1073/pnas.89.9.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine A J. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 8.Hollstein M, Sidransky D, Vogelstein B, Harris C C. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 9.Baker S J, Markowitz S J, Fearon E R, Willson J K V, Vogelstein B. Science. 1990;249:912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- 10.Harris C C, Hollstein M. N Engl J Med. 1993;329:1318–1327. doi: 10.1056/NEJM199310283291807. [DOI] [PubMed] [Google Scholar]
- 11.Vogelstein B, Kinzler K W. Cell. 1992;70:523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- 12.Cho Y, Gorina S, Jeffrey P D, Pavletich N P. Science. 1994;265:346–355. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- 13.Jeffrey P D, Gorina S, Pavletich N P. Science. 1995;267:1498–1502. doi: 10.1126/science.7878469. [DOI] [PubMed] [Google Scholar]
- 14.Lowe S W, Ruley H E, Jacks T, Housman D E. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 15.Lu X, Lane D P. Cell. 1993;75:765–778. doi: 10.1016/0092-8674(93)90496-d. [DOI] [PubMed] [Google Scholar]
- 16.Zhan Q, Fan S, Bae I, Guillouf C, Liebermann D A, O’Connor P M, Fornace A J., Jr Oncogene. 1994;9:3743–3751. [PubMed] [Google Scholar]
- 17.Kastan M B, Onyekwere O, Sidransky D, Vogelstein B, Craig R W. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 18.Kastan M B, Zhan Q, El-Diery W S, Carrier F, Jacks T, Walsh W V, Plunkett B S, Vogelstein B, Fornace A J., Jr Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 19.Kuerbitz S J, Plunkett B S, Walsh W V, Kastan M B. Proc Natl Acad Sci USA. 1992;89:7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tishler R B, Calderwood S K, Coleman C N, Price B D. Cancer Res. 1993;53:2212–2216. [PubMed] [Google Scholar]
- 21.El-Diery W S, Tokino S T, Valculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. Cell. 1994;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto K, Beach D. EMBO J. 1994;13:4816–4822. doi: 10.1002/j.1460-2075.1994.tb06807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mommand J, Zambetti G P, Olson D C, George D, Levine A J. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 24.Wu X, Bayle J H, Olson D, Levine A J. Genes Dev. 1993;7:1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- 25.Zambetti G P, Bargonetti J, Walker K, Prives C, Levine A J. Genes Dev. 1992;6:1143–1152. doi: 10.1101/gad.6.7.1143. [DOI] [PubMed] [Google Scholar]
- 26.Ginsberg D, Mechta F, Yaniv M, Oren M. Proc Natl Acad Sci USA. 1991;88:9979–9983. doi: 10.1073/pnas.88.22.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kley N, Chung R Y, Fay S, Loeffler J P, Seizinger B R. Nucleic Acids Res. 1992;20:4083–4087. doi: 10.1093/nar/20.15.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chin K V, Ueda K, Pastan I, Gottesman M M. Science. 1992;255:459–462. doi: 10.1126/science.1346476. [DOI] [PubMed] [Google Scholar]
- 29.Agoff S N, Hou J, Linzer D I H, Wu B. Science. 1993;259:84–87. doi: 10.1126/science.8418500. [DOI] [PubMed] [Google Scholar]
- 30.Santhanam U, Ray A, Sehgal P B. Proc Natl Acad Sci USA. 1991;88:7605–7609. doi: 10.1073/pnas.88.17.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subler M A, Martin D W, Deb S. J Virol. 1992;66:4757–4762. doi: 10.1128/jvi.66.8.4757-4762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris L C, Remack J S, Houghton P J, Brent T P. Cancer Res. 1996;56:2029–2032. [PubMed] [Google Scholar]
- 33.Liu X, Miller C W, Koeffler P H, Berk A J. Mol Cell Biol. 1993;13:3291–3300. doi: 10.1128/mcb.13.6.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mack D H, Vartikar J, Pipas J M, Laimins L A. Nature (London) 1993;363:281–283. doi: 10.1038/363281a0. [DOI] [PubMed] [Google Scholar]
- 35.Seto E, Usheva A, Zambetti G P, Momand J, Horikoshi N, Wienmann R, Levine A J, Shenk T. Proc Natl Acad Sci USA. 1992;89:12028–12032. doi: 10.1073/pnas.89.24.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borellini F, Glazer R I. J Biol Chem. 1993;268:7923–7928. [PubMed] [Google Scholar]
- 37.Spindler S A, Sarkar F H, Sakr W A, Blackburn M L, Bull A W, LaGattuta M, Reddy R G. Biochem Biophys Res Commun. 1997;239:775–781. doi: 10.1006/bbrc.1997.7471. [DOI] [PubMed] [Google Scholar]
- 38.Yin Y, Tainsky M A, Bischoff F Z, Strong L C, Wahl G M. Cell. 1992;70:937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- 39.Harvey D M, Levine A J. Genes Dev. 1991;5:2375–2385. doi: 10.1101/gad.5.12b.2375. [DOI] [PubMed] [Google Scholar]
- 40.Zambetti G P, Quartin R S, Martinez J, Georgoff I, Momand J, Dittmer D, Finlay C A, Levine A J. Cold Spring Harbor Symp Quant Biol. 1991;56:219–225. doi: 10.1101/sqb.1991.056.01.027. [DOI] [PubMed] [Google Scholar]
- 41.Moody T W, Leyton J, Martinez A, Hong S, Malkinson A, Mulshine J L. Exp Lung Res. 1998;24:617–628. doi: 10.3109/01902149809087390. [DOI] [PubMed] [Google Scholar]
- 42.Livingstone L R, White A, Sprouse J, Livanos E, Jacks T, Tlsty T D. Cell. 1992;70:923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- 43.Hussey H J, Tisdale M J. Br J Cancer. 1997;75:845–849. doi: 10.1038/bjc.1997.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ikawa H, Kamitani H, Calvo B F, Foley J F, Eling T E. Cancer Res. 1999;59:360–366. [PubMed] [Google Scholar]
- 45.Tang D G, Guan K L, Li L, Honn K V, Chen Y Q, Rice R L, Taylor J D, Porter A T. Int J Cancer. 1997;72:1078–1087. doi: 10.1002/(sici)1097-0215(19970917)72:6<1078::aid-ijc24>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 46.Tang D G, Honn K V. Adv Exp Med Biol. 1997;400A:349–361. doi: 10.1007/978-1-4615-5325-0_48. [DOI] [PubMed] [Google Scholar]
- 47.Rice R L, Tang D G, Haddad M, Honn K V, Taylor J D. Int J Cancer. 1998;77:271–278. doi: 10.1002/(sici)1097-0215(19980717)77:2<271::aid-ijc17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 48.Hussey H J, Tisdale M J. Br J Cancer. 1996;74:683–687. doi: 10.1038/bjc.1996.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelavkar U, Wang S, Montero A, Murtagh J, Shah K, Badr K. Mol Biol Rep. 1998;25:173–182. doi: 10.1023/a:1006813009006. [DOI] [PubMed] [Google Scholar]
- 50.Kern S E, Kinzler K W, Baker S J, Nigro J M, Rotter V, Levine A J, Friedman P, Prives C, Vogelstein B. Oncogene. 1991;6:131–136. [PubMed] [Google Scholar]
- 51.Muller B F, Paulsen D, Deppert W. Oncogene. 1996;12:1941–1952. [PubMed] [Google Scholar]
- 52.Thukral S K, Lu Y, Blain G C, Harvey T S, Jacobsen V L. Mol Cell Biol. 1995;15:5196–5202. doi: 10.1128/mcb.15.9.5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rolley N, Butcher S, Milner J. Oncogene. 1995;11:763–770. [PubMed] [Google Scholar]
- 54.Martinez J D, Craven M T, Pennington M E. Oncogene. 1998;16:453–458. doi: 10.1038/sj.onc.1201565. [DOI] [PubMed] [Google Scholar]
- 55.Deb S, Jackson C T, Subler M A, Martin D W. J Virol. 1992;66:6164–6170. doi: 10.1128/jvi.66.10.6164-6170.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Werner H, Karnieli E, Rauscher F J, LeRioth D. Proc Natl Acad Sci USA. 1996;93:8318–8323. doi: 10.1073/pnas.93.16.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Albor A, Kaku S, Kulesz-Martin M. Cancer Res. 1998;58:2091–2094. [PubMed] [Google Scholar]
- 58.Moll U M, LaQuaglia M, Benard J, Riou G. Proc Natl Acad Sci USA. 1995;92:4407–4411. doi: 10.1073/pnas.92.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu B, Timar J, Howlett J, Diglio C A, Honn K V. Cell Regul. 1991;12:1045–1055. doi: 10.1091/mbc.2.12.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Leyen K, Duvoisin R M, Engelhardt H, Wiedmann M. Nature (London) 1998;395:392–395. doi: 10.1038/26500. [DOI] [PubMed] [Google Scholar]