The testing and release of genetically modified organisms (GMOs)—in particular GM plants—is tightly regulated internationally to prevent any negative effects on the environment or human health. However, these regulations are based on transgenic organisms and do not discriminate between transgenic plants and cisgenic plants, although we believe that they are fundamentally different (see sidebar). Now, cisgenic plants fall under regulations designed for transgenic organisms, possibly because there have not yet been any applications for the approval of the deliberate release of cisgenic plants into the environment.
Although transgenesis and cisgenesis both use the same genetic modification techniques—namely the introduction of one or more genes and their promoters into a plant—cisgenesis involves only genes from the plant itself or from a close relative, and these genes could also be transferred by traditional breeding techniques. If the current international GMO regulations, which are mainly based on the process of transferring transgenes, continue to fail to differentiate between cisgenic and transgenic plants, the use of cisgenesis could be seriously hindered. Only Canada now has a product-based rather than a process-based regulation system, and therefore has the legal possibility to control cisgenic plants less strictly than transgenic plants. Any restrictions on cisgenesis could block or delay further research on improving crop varieties, particularly as an increasing number of functional genes from crops and their crossable wild relatives are being isolated and are becoming amenable to cisgenesis. We argue that cisgenic plants are fundamentally different from transgenic plants, and should therefore be treated differently under GMO regulations.
If the current international GMO regulations … continue to fail to differentiate between cisgenic and transgenic plants, the use of cisgenesis could be seriously hindered
In the case of transgenesis, the transferred gene usually derives from an alien species that is neither the recipient species nor a close, sexually compatible relative. In other words, transgenesis can extend the gene pool of the recipient species. Such a novel gene might provide the target plant with a new trait that neither occurs in the recipient species in nature nor can be introduced through traditional breeding. This novel trait might affect the fitness of the recipient species in various ways; a change in fitness can then spread through gene flow between a GM crop and its wild relatives (den Nijs et al, 2004), potentially creating shifts in natural vegetation. Consequently, lawmakers and regulatory authorities have paid much attention to the safety of deliberate releases of transgenic crops into the environment and have put in place biosafety frameworks to control this risk.
In the case of a cisgenic plant, the gene of interest, together with its promoter, has been present in the species or in a sexually compatible relative for centuries. Therefore cisgenesis does not alter the gene pool of the recipient species and provides no additional traits. No changes in fitness occur that would not happen through either traditional breeding or natural gene flow. Similarly, cisgenesis carries no risks—such as effects on non-target organisms or soil ecosystems, toxicity or a possible allergy risk for GM food or feed—other than those that are also incurred by traditional breeding. This is the fundamental difference between cisgenesis and transgenesis. Consequently, the deliberate release and market introduction of cisgenic plants is as safe as the release and market introduction of traditionally bred plants. On the issue of safety, regulators could treat cisgenic plants the same as conventionally bred plants (Schouten et al, 2006).
On the issue of safety, regulators could treat cisgenic plants the same as conventionally bred plants
Indeed, cisgenesis has great potential to overcome a major bottleneck in traditional breeding. During introgression breeding, a wild plant with an interesting trait is crossed with a high-quality genotype, such as a cultivar. The wild plant, however, passes on not only its genes of interest to the progeny, but also other, sometimes deleterious, genes. This so-called linkage drag can slow down the breeding process tremendously, especially if the gene of interest is genetically tightly linked to one or more deleterious genes. To reduce linkage drag, plant breeders usually need successive generations of recurrent backcrossing with the cultivated plant and simultaneous selection for the trait to generate a genotype in which the gene of interest is no longer linked to any undesired genes. By contrast, cisgenesis isolates only the gene of interest from the donor plant, which is then inserted into the recipient in one step. As no other genes are transferred, this method avoids linkage drag. This can enhance the breeding speed, particularly if several genes from different relatives must be combined into an elite variety, for example to obtain durable multigenic resistance. This is the main advantage of cisgenesis compared with traditional introgression breeding.
Cisgenesis is a particularly efficient method for cross-fertilizing heterozygous plants that propagate vegetatively, such as potato, apple and banana. It can directly improve an existing variety without disturbing the genetic make-up of the plant. Traditional introgression breeding of cross-fertilizing plants does not allow the introduction of genes from wild germplasm without mixing up the combination of alleles in the existing heterozygous elite recipient genotype.
One example is the introduction of the apple scab resistance gene Vf from a wild into a cultivated apple, which began as early as the 1950s (Hough et al, 1953; Schmidt & van de Weg, 2005). Despite more than 50 years of traditional breeding programmes, the new apple varieties carrying this gene have yet to acquire the same fruit quality in terms of taste and texture as the susceptible top varieties, because of linkage drag. As the Vf gene has recently been cloned (Belfanti et al, 2004), its transfer into elite varieties using cisgenesis could lead to better results in a considerably shorter time.
Similarly, breeding programmes aimed at rendering potatoes with durable resistance to the potato-late-blight-causing oomycete Phytophthora infestans require a series of genes from resistant wild species such as Solanum demissum and S. bulbocastanum. Introgression breeding with the new donor S. bulbocastanum began in the early 1970s, but has had only limited success because of linkage drag. In the meantime several natural resistance genes have been isolated from this donor and from S. demissum (Huang et al, 2005; van der Vossen et al, 2005), which would enable breeders to use cisgenesis to render existing susceptible elite potato varieties resistant by stacking cloned resistance genes.
The prerequisite for cisgenesis is the isolation and characterization of genes of interest from crossable relatives. The rapidly increasing amount of DNA sequence information for individual genes, multigene families and whole plant genomes, combined with our increasing knowledge of gene functions, has enabled a directed search for beneficial alleles among cultivated plants and their wild relatives. In the past decade, a large number of natural genes from crops and their wild relatives have been isolated, many of which code for important traits such as disease resistance and quality. Many of these genes are now sufficiently characterized and are ready to be transferred into elite crops.
However, whether this technique will develop into a powerful new tool strongly depends on several factors: how cisgenic plants are treated by existing legal frameworks (Bradford et al, 2005); consumer acceptance of such products; whether these plants and any products derived from them must be labelled as GM; and intellectual property rights on GM technologies and genes. Although intellectual property and consumer acceptance are largely beyond the control of lawmakers and regulators, it would be sensible to regulate cisgenic plants differently to transgenic plants.
Self-evidently, cisgenic plants should still be tested to confirm that they contain only the intended modifications and no foreign genes, such as a backbone gene from a plasmid. If such a foreign gene is unintentionally introduced, the plant is, by definition, transgenic.
Self-evidently, cisgenic plants should still be tested to confirm that they contain only the intended modifications and no foreign genes…
With regard to the species' gene pool, cisgenesis is equivalent to traditional breeding. However, there are differences, as recombinant DNA technology is certainly not the same as meiotic recombination. First, the donor sequence is inserted into the genome at an a priori unknown position, which might affect DNA methylation and other factors that in turn can influence gene expression. A biological counterargument is that translocations and (de)methylations also occur in nature. Lai et al (2005) showed that Helitron transposons in maize capture a 5.9 kilobase-long DNA fragment containing three genes and move it to another part of the maize genome. This is a natural process without any human intervention. In addition, a regulatory counterargument is the fact that traditional breeding also causes translocations of DNA fragments to previously unknown positions (Lin et al, 1999; Li et al, 2005). These might comprise large fragments, containing hundreds of genes. The fact that the location of the inserted gene is random is not a fundamental difference between cisgenesis and traditional breeding.
Second, the insertion of a cisgene results in a mutation at the insertion site. Moreover, rearrangements or translocations might occur in the flanking regions (Forsbach et al, 2003; Tax & Vernon, 2001). These mutations might knock out genes, open new reading frames and thereby induce phenotypic effects. But natural mutations and rearrangements in plant genomes are common, especially in chromosome regions where transposons are active. Unintended genome reorganization can also be induced by pathogen attack, abiotic stress and interspecies hybridization (Madlung & Comai, 2004). A regulatory counterargument is that, in Europe, mutation breeding is now exempt from the regulations on the release of GMOs into the environment (European Parliament, 2001). Usually, mutagenesis is achieved either by radiation or chemicals, both of which lead to random mutations and translocations. In general, mutagenesis causes larger changes at the DNA level compared with the changes that occur at the integration site of a cisgene or transgene (Shirley et al, 1992; Cecchini et al, 1998).
However, in the case of mutation breeding, current regulations do not require molecular characterization of all mutations in a plant before market introduction, and the nature and number of the mutations introduced are usually unknown. In the past 70 years, mutation breeding has led to more than 2,250 plant varieties, derived either as direct mutants or from their progenies (Ahloowalia et al, 2004). Although these mutation-derived plant varieties have been produced and used for food, feed or as ornamentals in more than 30 countries for several decades (Ahloowalia et al, 2004), we are not aware of any indications that the underlying mutations have caused damage to the environment, or had adverse effects on human or animal health (van Harten, 1998). This is circumstantial evidence that the phenotypic screening and selection process—the rule in plant breeding programmes—in combination with other conventional selection procedures before the introduction of new varieties onto the market, have been sufficient to reduce the risk of unknown mutations to an acceptably low level. For the development of cisgenic varieties, similar phenotypic screening and selection will be the rule. We can thus infer that cisgenesis and mutation breeding do not differ fundamentally with regard to unintended mutations.
Third, the donor sequence does not replace an allelic sequence, but is added to the recipient species' genome. Owing to the process of gene transfer, it is possible that the new sequence is inserted several times in one genome, which might affect gene expression and, therefore, phenotype. However, gene duplication is a common natural occurrence, for instance in the case of resistance genes or other multigene families (Bergelson et al, 2001). In fact, duplication is an integral part of the evolution of gene families. Furthermore, neither the increase of the ploidy level—a genome-wide DNA duplication that might affect the expression of numerous genes—nor the use of monosomic additions, are now considered in any GMO regulation (European Parliament, 2001), but have been applied for decades in plant breeding. The fact that the transferred cisgene is added to the recipient's genome is not fundamentally different from natural processes or traditional breeding techniques.
Fourth, the cisgenic plant might contain some small, non-coding sequences from the vector such as T-DNA borders, which are 25-base-pair imperfect repeats that delimit the DNA segment transferred to plant cells when using Agrobacterium-mediated gene transfer. Other non-coding sequences from the vector might be parts of a multiple cloning site or remnants from recombination sites that were used to excise undesired DNA sequences, such as a selection gene, after the DNA transfer (Schaart et al, 2004). However, these short DNA sequences are by nature non-coding and are unlikely to have a phenotypic effect. Moreover, some researchers have identified several DNA sequences within plants that are in essence identical to and can be used to functionally replace short vector sequences, such as T-DNA borders (Rommens et al, 2004; Conner et al, 2006). Consequently, the gene transfer process would introduce no alien DNA, not even non-coding foreign DNA. However, we believe that this is merely a semantic adaptation, rather than a means of controlling risk.
By definition, cisgenesis is a form of genetic modification, as it transfers a gene and its promoter to a recipient species. However, the product is clearly different from transgenic plants, which are derived by transferring ‘foreign' or artificial genes, or artificial combinations of genes and promoters. Cisgenesis therefore respects species barriers, and in this sense differs fundamentally from transgenesis. We argue that, for this reason, cisgenic plants are similar to traditionally bred plants, because the transferred genes come from the same gene pool. Consequently, cisgenic plants are as safe as traditionally bred plants.
Cisgenesis therefore respects species barriers, and in this sense differs fundamentally from transgenesis
Although the European legislative framework on GMOs regards mutagenesis and the fusion of cells from sexually compatible plants as methods of genetic modification, the resulting GM plants are excluded from that framework by the GMO Directive (European Parliament, 2001). Considering that the products of cisgenesis are more similar to plants derived by mutagenesis or traditional breeding methods, cisgenesis should also be excluded from GMO frameworks (Fig 1) and regulated in the same way as traditional breeding. Given the great potential that cisgenesis has to speed up the breeding process in plants, in particular to obtain durable multigenic resistance, such a decision would greatly enhance the economic and environmental prospects of agriculture.
Figure 1.

Annex 1 B of the GMO Directive 2001/18/EC (European Parliament, 2001). We propose adding the last sentence, to include cisgenesis.
Definitions of key terms in relation to plants.
Cisgenesis is the genetic modification of a recipient plant with a natural gene from a crossable—sexually compatible—plant. Such a gene includes its introns and is flanked by its native promoter and terminator in the normalsense orientation.Cisgenic plants can harbour one or more cisgenes, but they do not contain any transgenes.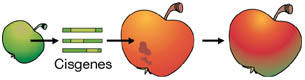
Transgenesis is the genetic modification of a recipient plant with one or more genes from any non-plant organism, or from a donor plant that is sexually incompatible with the recipient plant. This includes gene sequences of any origin in the anti-sense orientation, any artificial combination of a coding sequence and a regulatory sequence, such as a promoter from another gene, or a synthetic gene.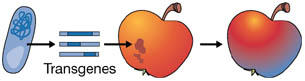
Traditional breeding encompasses all plant breeding methods that do not fall under current GMO regulations.As the European legal framework defines GMOs and specifies various breeding techniques that are excluded from the GMO regulations,we use this framework as a starting point, particularly the European Directive 2001/18/EC on the deliberate release of GMOs into the environment (European Parliament, 2001). Excluded from this GMO Directive are longstanding cross breeding, in vitro fertilization, polyploidy induction,mutagenesis and fusion of protoplasts from sexually compatible plants (European Parliament, 2001).

Henk J. Schouten

Frans A. Krens

Evert Jacobsen
Acknowledgments
This study was financially supported by TransForum, initiated and partly funded by the Dutch government to contribute to a more sustainable and innovative knowledge infrastructure in agriculture.
References
- Ahloowalia BS, Maluszynski M, Nichterlein K (2004) Global impact of mutation-derived varieties. Euphytica 135: 187–204 [Google Scholar]
- Belfanti E, Silfverberg-Dilworth E, Tartarini S, Patocchi A, Barbieri M, Zhu J, Vinatzer BA, Gianfranceschi L, Gessler C, Sansavini S (2004) The HcrVf2 gene from a wild apple confers scab resistance to a transgenic cultivated variety. Proc Natl Acad Sci USA 101: 886–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergelson J, Kreitman M, Stahl EA, Tian D (2001) Evolutionary dynamics of plant R-genes. Science 292: 2281–2285 [DOI] [PubMed] [Google Scholar]
- Bradford KJ, Van Deynze A, Gutterson N, Parrott W, Strauss SH (2005) Regulating transgenic crops sensibly: lessons from plant breeding, biotechnology and genomics. Nat Biotechnol 23: 439–444 [DOI] [PubMed] [Google Scholar]
- Cecchini E, Mulligan BJ, Covey SN, Milner JJ (1998) Characterization of gamma irradiation-induced deletion mutations at a selectable locus in Arabidopsis. Mutat Res 401: 199–206 [DOI] [PubMed] [Google Scholar]
- Conner AJ, Barrell PJ, Baldwin SJ, Lokerse AS, Cooper PA, Erasmuson AK, Nap JPH, Jacobs JME (2006) Intragenic vectors for gene transfer without foreign DNA. Euphytica (in press) [Google Scholar]
- den Nijs HCM, Bartsch D, Sweet J (2004) Introgression from Genetically Modified Plants into Wild Relatives. Wallingford, UK: CABI [Google Scholar]
- European Parliament (2001) Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EEC. Off J Eur Comm L 106: 1–38 [Google Scholar]
- Forsbach A, Schubert D, Lechtenberg B, Gils M, Schmidt R (2003) A comprehensive characterization of single-copy T-DNA insertions in the Arabidopsis thaliana genome. Plant Mol Biol 52: 161–176 [DOI] [PubMed] [Google Scholar]
- Hough LF, Shay JR, Dayton DF (1953) Apple scab resistance from Malus floribunda Sieb. Proc Amer Soc Hort Sci 62: 341–347 [Google Scholar]
- Huang S, van der Vossen EA, Kuang H, Vleeshouwers VG, Zhang N, Borm TJ, van Eck HJ, Baker B, Jacobsen E, Visser RG (2005) Comparative genomics enabled the isolation of the R3α late blight resistance gene in potato. Plant J 42: 251–261 [DOI] [PubMed] [Google Scholar]
- Lai J, Li Y, Messing J, Dooner HK (2005) Gene movement by Helitron transposons contributes to the haplotype variability of maize. Proc Natl Acad Sci USA 102: 9068–9073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chen X, Xin ZY, Ma YZ, Xu HJ, Chen XY, Jia X (2005) Development and identification of wheat-Haynaldia villosa T6DL.6VS chromosome translocation lines conferring resistance to powdery mildew. Plant Breeding 124: 203–205 [Google Scholar]
- Lin X et al. (1999) Sequence and analysis of chromosome 2 of the plant Arabidopsis thaliana. Nature 402: 761–768 [DOI] [PubMed] [Google Scholar]
- Madlung A, Comai L (2004) The effect of stress on genome regulation and structure. Ann Bot (Lond) 94: 481–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommens CM, Humara JM, Ye J, Yan H, Richael C, Zhang L, Perry R, Swords K (2004) Crop improvement through modification of the plant's own genome. Plant Physiol 135: 421–431 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Schaart JG, Krens FA, Pelgrom KTB, Mendes O, Rouwendal GJA (2004) Effective production of marker-free transgenic strawberry plants using inducible site-specific recombination and a bifunctional selectable marker gene. Plant Biotechnol J 2: 233–240 [DOI] [PubMed] [Google Scholar]
- Schouten HJ, Krens FA, Jacobsen E (2006). Do cisgenic plants warrant less stringent oversight? Nature Biotechnol (in press) [DOI] [PubMed] [Google Scholar]
- Schmidt H, van de Weg WE (2005) Breeding. In Tromp J, Webster AD, Wertheim SJ (eds) Fundamentals of Temperate Zone Tree Fruit Production pp 136–155. Leiden, The Netherlands: Backhuys [Google Scholar]
- Shirley BW, Hanley S, Goodman HM (1992) Effects of ionizing radiation on a plant genome: analysis of two Arabidopsis transparent testa mutations. Plant Cell 4: 333–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tax FE, Vernon DM (2001) T-DNA-associated duplication/translocations in Arabidopsis. Implications for mutant analysis and functional genomics. Plant Physiol 126: 1527–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vossen EA, Gros J, Sikkema A, Muskens M, Wouters D, Wolters P, Pereira A, Allefs S (2005) The Rpi-blb2 gene from Solanum bulbocastanum is an Mi-1 gene homolog conferring broad-spectrum late blight resistance in potato. Plant J 44: 208–222 [DOI] [PubMed] [Google Scholar]
- van Harten AM (1998) Mutation Breeding: Theory and Practical Applications. Cambridge, UK: Cambridge University Press [Google Scholar]



