Abstract
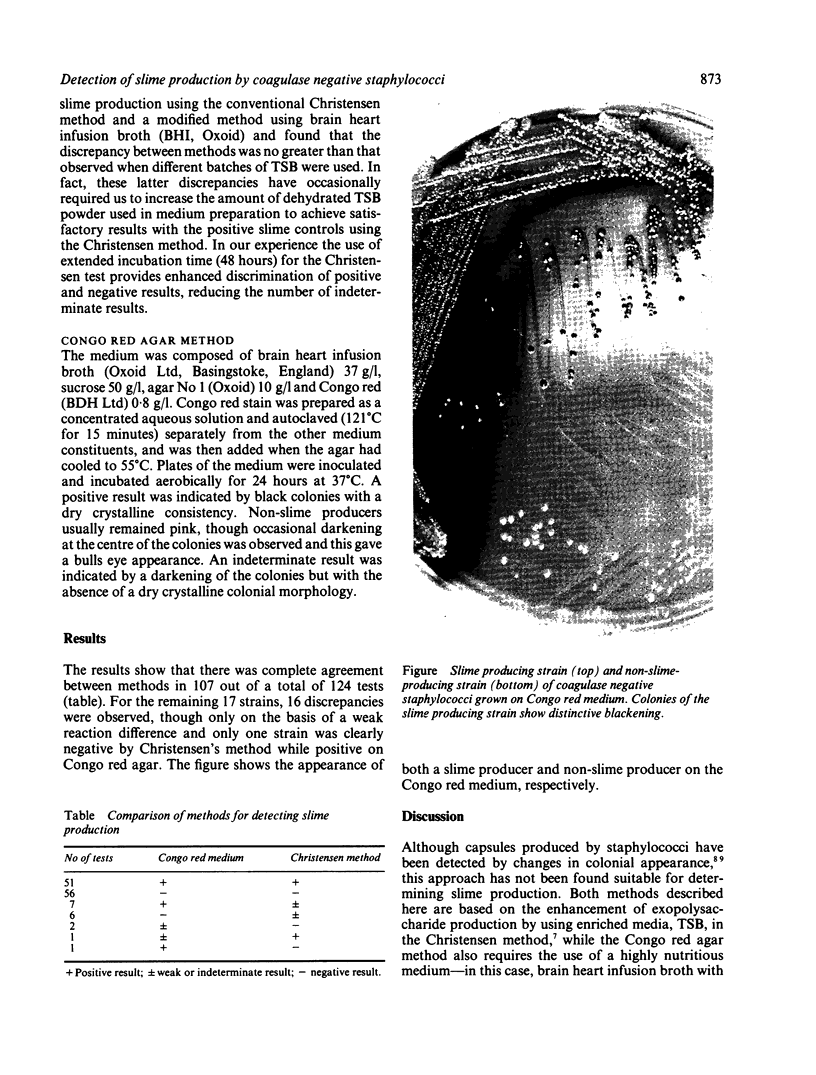
An alternative method for detecting the production of slime by coagulase negative staphylococci was compared with the routinely used Christensen method on 124 isolates of coagulase negative staphylococci from carriage sites, blood cultures, and infected peritoneal dialysis fluids. The alternative method requires the use of a specially prepared solid medium--brain heart infusion broth, supplemented with 5% sucrose, and Congo red stain. Of the 124 tests, there was complete agreement between methods in 107 and only one strain was clearly negative by Christensen's method while positive on Congo red agar. The Congo red method is rapid, sensitive, and reproducible and has the advantage that colonies remain viable on the medium. It is also not subject ot interbatch variation of media which sometimes affects the reproducibility of the Christensen method.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayston R., Penny S. R. Excessive production of mucoid substance in staphylococcus SIIA: a possible factor in colonisation of Holter shunts. Dev Med Child Neurol Suppl. 1972;27:25–28. doi: 10.1111/j.1469-8749.1972.tb09769.x. [DOI] [PubMed] [Google Scholar]
- Blackshaw A. J., Levison D. A. Eosinophilic infiltrates of the gastrointestinal tract. J Clin Pathol. 1986 Jan;39(1):1–7. doi: 10.1136/jcp.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982 Jul;37(1):318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishak M. A., Gröschel D. H., Mandell G. L., Wenzel R. P. Association of slime with pathogenicity of coagulase-negative staphylococci causing nosocomial septicemia. J Clin Microbiol. 1985 Dec;22(6):1025–1029. doi: 10.1128/jcm.22.6.1025-1029.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. M., Lee D. A., Regelmann W. E., Gray E. D., Peters G., Quie P. G. Interference with granulocyte function by Staphylococcus epidermidis slime. Infect Immun. 1986 Oct;54(1):13–20. doi: 10.1128/iai.54.1.13-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Pulverer G. Pathogenesis and management of Staphylococcus epidermidis 'plastic' foreign body infections. J Antimicrob Chemother. 1984 Dec;14 (Suppl 500):67–71. doi: 10.1093/jac/14.suppl_d.67. [DOI] [PubMed] [Google Scholar]
- Smith R. M., Parisi J. T., Vidal L., Baldwin J. N. Nature of the genetic determinant controlling encapsulation in Staphylococcus aureus Smith. Infect Immun. 1977 Jul;17(1):231–234. doi: 10.1128/iai.17.1.231-234.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger J. J., Christensen G. D., Bartley D. L., Simmons J. C., Barrett F. F. Coagulase-negative staphylococci isolated from cerebrospinal fluid shunts: importance of slime production, species identification, and shunt removal to clinical outcome. J Infect Dis. 1987 Oct;156(4):548–554. doi: 10.1093/infdis/156.4.548. [DOI] [PubMed] [Google Scholar]




