Abstract
Mouse cytotoxic T lymphocytes (CTL) reactive with a H-2Db-presented 9-mer peptide of the human papillomavirus type 16 protein E749-57 (RAHYNIVTF) were generated from the spleen cells of wild-type C57BL/6 (B6) or B6 perforin-deficient (B6.P0) mice. CD8+ B6 CTL displayed peptide-specific perforin- and Fas-mediated lysis of E7-transfected mouse RMA lymphoma cells (RMA-E7), while CD8+ CTL from B6.P0 mice lysed RMA-E7 cells via Fas ligand (FasL) exclusively. Rapid and efficient lysis of syngeneic bystander B6 blasts or RMA cells by either B6 or B6.P0 Ag-activated CTL was mediated by a FasL-Fas mechanism. Fas-resistant bystanders were not lysed, nor were allogeneic Fas-sensitive C3H/HeJ (H-2k) or BALB/c (H-2d) bystander blasts. Interestingly, however, phorbol myristate acetate-ionomycin preactivation of B6.P0 effectors enabled lysis of allogeneic H-2k and H-2d bystanders even in the absence of antigenic stimulation. Lysis of syngeneic bystander cells was always FasL-Fas dependent and required effector-bystander contact and, in particular, an interaction between CTL LFA-1 and bystander ICAM-1. Thus, in the context of major histocompatibility complex class I molecule-peptide ligation of the T-cell receptors of CD8+ CTL, neighboring bystander cells that are syngeneic and Fas sensitive and express the adhesion molecule ICAM-1 are potential targets of CTL attack.
With the dissection of two basic cytolytic mechanisms of cytotoxic T lymphocytes (CTL) (10, 14, 20, 34), it has become possible to delineate the important criteria that determine direct (Ag-restricted) and bystander cytotoxicity. CTL use complementary cytotoxic mechanisms, one based on the granule exocytosis of a calcium-dependent pore-forming protein, perforin (8, 26), and granzymes (35) and another that depends on a calcium-independent interaction of effector T-cell tumor necrosis factor or Fas ligand (TNF or FasL) and target cell TNF receptor (TNFR) or Fas (22, 33). The function of the granule exocytosis pathway appears to be largely in non-major histocompatibility complex (MHC)-restricted NK lysis of class I molecule-defective tumor cells and in direct CTL-mediated immunity against tumor cells (37) or virus-infected cells (11, 19, 39). By contrast, the FasL-Fas and TNF-TNFR interactions are important for the maintenance of T-cell homeostasis following exposure to foreign Ag (5, 42) and Th-1 FasL-mediated B-cell apoptosis (27, 28). Blockage of both TNF and FasL is required to abrogate T-cell death: TNF mediates the death of most CD8+ T cells, whereas FasL mediates the death of most CD4+ T cells (42). While FasL-dependent lysis appears to be the primary mechanism used by CD4+ Th-1 effectors, CD8+ CTL use FasL or TNF secondarily in the absence of perforin-mediated lysis (10, 14, 20).
After T-cell activation, a functional role for FasL is not apparent for several days until the T cell becomes Fas sensitive and hence susceptible to autocrine T-cell suicide (1, 5, 38). However, by using alloreactive CTL cultures or clones, it has recently become apparent that in the presence of Ag-bearing target cells (i.e., upon T-cell receptor [TCR] activation) CTL can also lyse Ag-free bystander cells via a FasL-Fas interaction (13, 34). While the specificity of CTL toward Ag-bearing target cells has been considered a hallmark of an efficient immune response, CTL do not appear to spare Ag-free bystander cells during lysis of specific Ag-bearing target cells. In this study, we have generated CD8+ CTL from both wild-type and perforin-deficient (P0) mice reactive with a high-affinity H-2Db-binding peptide of human papillomavirus type 16 protein E7. These peptide-specific CTL have been employed to demonstrate the requirements for CD8+ CTL-mediated lysis of Ag-free bystander cells and in particular the different properties of CTL activated by antigen versus a nonspecific stimulus.
MATERIALS AND METHODS
Mice.
C57BL/6.P0 (B6.P0) mice derived as described previously (11) were obtained from Gary Kupariah, John Curtin School of Medical Research, Canberra, Australia. C57BL/6 ICAM-1-deficient (B6.ICAM0) mice (32) were obtained from The Jackson Laboratories, Bar Harbor, Maine. These strains and B6 β2μ-deficient (B6.β2μ0) mice were maintained and bred at the Austin Research Institute Biological Research Laboratories. C3H/HeJ, BALB/c, and B6 mice were purchased from The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia.
Cells and reagents.
The mouse EL4 (H-2b) thymoma, RMA (H-2b) lymphoma, and RMA-S (H-2b) mutant lymphoma (derived from the Raucher virus-induced murine cell line RBL-5 and defective for peptide loading of MHC class I molecules) (18) cell lines and an E7-transfected clone of RMA, RMA-E7 (43), were grown in RPMI medium supplemented with 10% (vol/vol) fetal calf serum (FCS), 2 mM glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin (Gibco, Grand Island, N.Y.)/ml. d12S, the 12th serial subcloning of the rat × mouse hybridoma PC60 cell line (3), was grown in Dulbecco’s modified Eagle’s medium (Gibco) supplemented with 5% FCS and additives as described above. Phorbol myristate acetate (PMA) (Sigma Chemical Co., St. Louis, Mo.) and ionomycin (ION) (Calbiochem Corp., San Diego, Calif.) were purchased. Soluble recombinant human FasL (srFasL) (produced in COS cell supernatants and used neat), mouse Fas-Fc, and human p80 TNFR-Fc fusion proteins were a kind gift from David Lynch, Immunex Corp., Seattle, Wash. Recombinant human interleukin-2 (IL-2) was a kind gift from Chiron Corp., Emeryville, Calif. Peptides of human papillomavirus type 16 protein E749-57 (RAHYNIVTF) and chicken OVA257-264 (SIINFEKL) were synthesized (>98% pure) on an Applied Biosystems model 430A automated peptide synthesizer (12). d12S cells were incubated for 3 h before the cytotoxicity tests with a mixture of PMA (5 ng/ml) and ION (1 μg/ml). Spleen cells were harvested from B6, B6.ICAM0, C3H/HeJ (H-2k), and BALB/c (H-2d) mice and cultured in RPMI medium supplemented with the additives described above and ION (1 μg/ml) and PMA (5 ng/ml) for 3 days. Spleen blasts were then washed three times in medium prior to their use as target cells. Spleen blasts were >98% CD3+ as determined by flow cytometry.
Peptide immunization and induction of B6 and B6.P0 anti-E749-57 CTL.
One hundred micrograms (100 μl of 1 mg/ml peptide solution) of peptide was extensively mixed with 100 μl of immunofluorescent antibody and 0.5% bovine serum albumin. The 200-μl mixture was injected subcutaneously into B6 or B6.P0 mice, and the procedure was repeated after 2 weeks. Two weeks after the second immunization the mice were sacrificed and spleen cells were cultured overnight on plastic flasks to remove adherent cells. Responder cells were then treated with anti-CD4 (H129.19; rat immunoglobulin G2a [IgG2a]) (Sigma) and complement (1/30 dilution of normal rabbit serum) as previously described (36). Stimulator RMA-S cells were cultured at 25°C for 24 h and incubated with 100 μM E749-57 peptide for 2 to 4 h at 33°C. Stimulator cells (106) were extensively washed, irradiated (20,000 rad), and then cultured (25-cm2 culture flasks) with responder cells (2 × 107) in RPMI supplemented with 10% FCS and 5 μM peptide. After 5 days, responder lymphocytes were harvested and cultured in 24-well plates at a density of 106 cells per well in the same medium supplemented with 5 U of IL-2/ml. Each well received 106 irradiated RMA-E7 cells as stimulator cells. Responder cells were restimulated weekly under the same conditions; they were >90% CD8+ as determined by immunofluorescence assay, and their CTL activity was determined in a 4-h 51Cr release assay with RMA-E7 target cells.
51Cr release assays.
The cytotoxicity of responding B6 anti-E749-57 or B6.P0 anti-E749-57 CTL, d12S cells, or srFasL was assessed by 51Cr release assays for 4 h as described previously (30). Briefly, Ag-bearing target cells (including temperature-induced RMA-S cells that were pulse labeled with immunizing or control peptide, as described above) or Ag-free bystander cells were labeled with 50 μCi of 51Cr for 1 h at 37°C and then washed three times. 51Cr-labeled Ag-bearing target cells (104) and unlabeled bystander cells (104) were incubated with different numbers of CTL as indicated, according to the effector/target cell ratio. In other wells, 51Cr-labeled Ag-free cells and unlabeled Ag-bearing target cells (also at a 1:1 ratio) were added to CTL. The spontaneous release of 51Cr was determined by incubating the target cells with medium alone, whereas the maximum release was determined by adding sodium dodecyl sulfate to a final concentration of 5%. The percent specific lysis was calculated as follows: 100 × [(experimental release − spontaneous release)/(maximum release − spontaneous release)]. In some experiments, B6 or B6.P0 anti-E749-57 CTL were incubated for 3 h before the cytotoxicity tests with a mixture of PMA (5 ng/ml) and ION (1 μg/ml). These cells were washed three times prior to the assay. In other experiments, B6 or B6.P0 CTL were depleted with monoclonal antibody (MAb) (10 μg/ml; anti-CD8 [1803; rat IgG2a] or control anti-CD4 and complement [1/30 dilution of normal rabbit serum]) prior to the examination of cytolysis against RMA-E7 and bystander syngeneic target cells. Cytotoxicity tests were sometimes performed either in the absence or presence of Fas-Fc or TNFR-Fc (at a final concentration of 5 μg/ml) and/or EGTA (Sigma) (at a final concentration of 2.5 mM). In other tests MAb (anti-LFA-1-α [M17/4; rat IgG2a] [Pharmingen, San Diego, Calif.], anti-CD8 [53-6.7; rat IgG2a] [Sigma], or anti-Thy 1.1 [OX-7; mouse IgG1]) was added at a final concentration of 20 μg/ml prior to, or 1 h after, the commencement of the cytotoxicity assay.
RESULTS AND DISCUSSION
Generation of E749-57-specific B6 and B6.P0 CD8+ CTL.
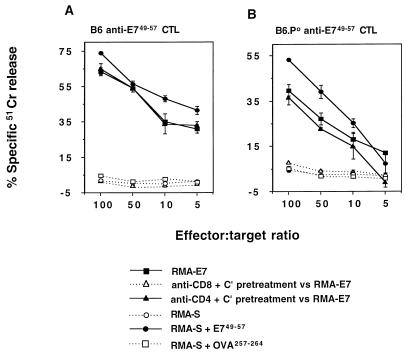
Following immunization of B6 and B6.P0 mice with E749-57 peptide, harvested spleen cells were depleted of CD4+ T cells and responding CTL were expanded by several rounds of stimulation with irradiated RMA-E7 cells. Resultant B6 and B6.P0 CTL cultures were assayed for specific cytolytic activity towards RMA-E7 or RMA-S cells pulsed with the E749-57 (RAHYNIVTF) peptide in a 4-h 51Cr release assay (Fig. 1). RMA-S cells have a defect affecting peptide loading of MHC class I molecules, and as a consequence, the cell surface expression of these molecules is low but increases when Kb- or Db-binding peptides are added to the culture medium (18). Both B6 and B6.P0 CTL cultures effectively lysed RMA-E7 or RMA-S cells pulsed with E749-57 but not RMA-S cells pulsed with OVA257-264 or RMA-S cells alone. From several independent immunizations and in vitro cultures, peptide-specific lysis by B6 CTL was consistently greater than that by B6.P0 CTL. Pretreatment of these cultures with anti-CD8 MAb and complement, but not with control anti-CD4 MAb and complement, completely abrogated B6 anti-E749-57 or B6.P0 anti-E749-57 CTL lysis of RMA-E7 (Fig. 1). Overall, these data indicate that the cytotoxic effects studied were mediated by CD8+ CTL and were specific for E749-57 peptide.
FIG. 1.
Generation of E749-57-specific B6 and B6.P0 CD8+ CTL. Mice were immunized with E749-57 peptide, and responding CTL were induced as described in Materials and Methods. The resultant B6 (A) and B6.P0 (B) CTL cultures were assayed for specific cytolytic activity toward RMA-E7 cells, RMA-S cells alone, or RMA-S cells pulsed with the E749-57 peptide or the OVA257-264 peptide in a 4-h 51Cr release assay. Some effectors were pretreated with anti-CD8 MAb and complement or anti-CD4 MAb and complement prior to examination of the cytolysis of RMA-E7 target cells. Direct cytotoxicity was assessed at the four effector/target ratios illustrated (104 target cells/well). Cytotoxicity was expressed as specific 51Cr release after subtraction of spontaneous 51Cr release, which was less than 15%. These results were calculated as the means ± standard errors of duplicate samples and are representative of three experiments performed.
B6 and B6.P0 CD8+ CTL specific for E749-57 mediate bystander lysis.
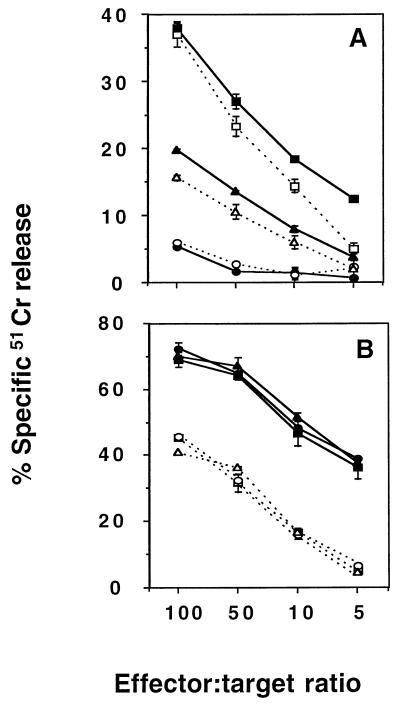
Figure 2 demonstrates that E749-57-specific CTL from B6 or B6.P0 mice could efficiently lyse Ag-free noncognate targets when the latter were bystanders to lysis of Ag-bearing RMA-E7 target cells. In particular, syngeneic (H-2b) B6 blasts and, to a lesser extent, RMA cells acted as susceptible bystanders, while syngeneic (H-2b) EL4 cells did not (Fig. 2A). These data correlated with the relative Fas sensitivity of these bystander cells (B6 blasts were more sensitive to FasL than RMA cells, and EL4 cells were insensitive [see Fig. 5]). The levels of bystander lysis mediated by B6 and B6.P0 CTL specific for E749-57 were approximately equivalent. B6 anti-E749-57 or B6.P0 anti-E749-57 CTL lysed RMA-E7 cells irrespective of the type of bystander cell present (Fig. 2B).
FIG. 2.
B6 and B6.P0 CD8+ CTL specific for E749-57 mediate bystander lysis. (A) Bystander lysis by B6 (solid symbols) and B6.P0 (open symbols) CD8+ CTL specific for E749-57 was examined at the four effector/target ratios illustrated (104 51Cr-labeled bystanders and 104 cold RMA-E7 cells/well) against 51Cr-B6 blasts and RMA-E7 cells (squares), 51Cr-EL4 and RMA-E7 cells (circles), or 51Cr-RMA and RMA-E7 cells (triangles) in a 4-h 51Cr release assay. Bystander lysis of B6 blasts or EL4 cells was not observed in the absence of RMA-E7 cells or presence of RMA cells (<10% lysis at all effector/target ratios). (B) Direct lysis by B6 (solid symbols) and B6.P0 (open symbols) CD8+ CTL specific for E749-57 was examined at the four effector/target ratios illustrated (104 51Cr-labeled RMA-E7 target cells and 104 cold bystanders/well) against 51Cr-RMA-E7 cells and B6 blasts (squares), 51Cr-RMA-E7 and EL4 cells (circles), or 51Cr-RMA-E7 and RMA cells (triangles) in a 4-h 51Cr release assay. Cytotoxicity was expressed as specific 51Cr release after subtraction of spontaneous 51Cr release, which was <15%. These results were calculated as the means ± standard errors of duplicate samples and are representative of two experiments performed.
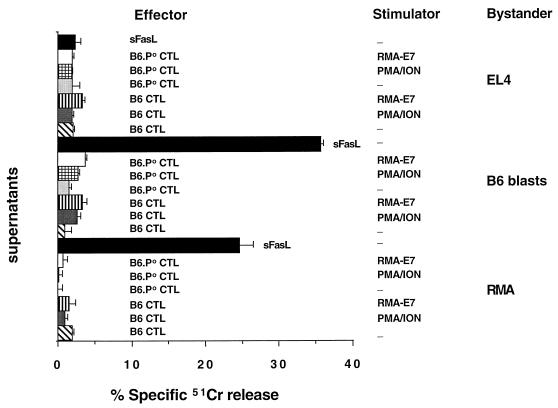
FIG. 5.
No FasL-mediated activity in culture supernatants from activated CTL. Anti-E749-57 CTL from B6 and B6.P0 mice (effectors) were stimulated with PMA-ION or incubated with Ag-bearing RMA-E7 cells (stimulators) as for a 4-h assay (effector/target ratio of 50:1). Supernatants were collected at 4 h and evaluated for their ability to lyse 51Cr-labeled B6 blasts, RMA cells (Fas sensitive), or EL4 cells (Fas insensitive) in a 4-h 51Cr release assay. srFasL (sFasL) was used as a positive control. Cytolysis was expressed as specific 51Cr release after subtraction of spontaneous 51Cr release, which was <15%. These results were calculated as the means ± standard errors of duplicate samples.
Bystander lysis by E749-57-specific B6 or B6.P0 CD8+ CTL is FasL mediated.
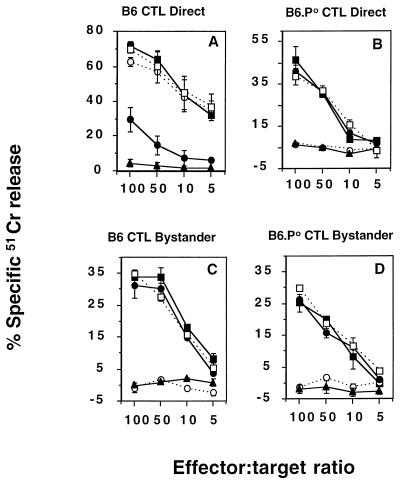
The cytolytic mechanisms employed by B6 and B6.P0 CD8+ CTL specific for E749-57 were determined by the addition of an inhibitory Fas-Fc fusion protein and/or the Ca2+ chelator, EGTA (to block granule-mediated lysis). Direct lysis of RMA-E7 target cells by B6 CTL specific for E749-57 was not inhibited by Fas-Fc, was significantly inhibited by EGTA, but was completely inhibited by a combination of Fas-Fc and EGTA (Fig. 3A). B6 CTL inhibited from lysing RMA-E7 targets (by the addition of EGTA) still efficiently killed bystander B6 blasts. Thus, B6 CTL specific for E749-57 lysed RMA-E7 target cells preferentially via granule exocytosis, but in the absence of granule exocytosis by FasL. By contrast, Fas-Fc alone completely blocked B6.P0 anti-E749-57-mediated lysis of RMA-E7, and EGTA was without effect (Fig. 3B), suggesting that these P0 CTL mediated Ag-specific lysis via FasL. A potential nonspecific inhibition of lysis by the Fas-Fc fusion protein was unlikely given that a control TNFR-Fc fusion protein at the same concentration was ineffective. Bystander lysis of B6 blasts in the presence of RMA-E7, mediated by either B6 (Fig. 3C) or B6.P0 (Fig. 3D) CTL specific for E749-57, was inhibited by Fas-Fc alone but not by TNFR-Fc or EGTA.
FIG. 3.
Bystander lysis by E749-57-specific B6 or B6.P0 CD8+ CTL is FasL mediated. Direct lysis by B6 (A) and B6.P0 (B) CTL specific for E749-57 (at the four effector/target ratios illustrated; 104 51Cr-labeled RMA-E7 target cells and 104 cold B6 blasts/well) and bystander lysis by B6 (C) and B6.P0 (D) CTL specific for E749-57 (at the four effector/target ratios illustrated; 104 51Cr-labeled B6 blasts and 104 cold RMA-E7 cells/well) were examined in a 4-h 51Cr release assay. These assays were performed in the absence (solid squares) or presence of Fas-Fc (open circles), EGTA (solid circles), Fas-Fc and EGTA (solid triangles), or TNFR-Fc (open squares). Cytotoxicity was expressed as specific 51Cr release after subtraction of spontaneous 51Cr release, which was <15%. These results were calculated as the means ± standard errors of duplicate samples and are representative of two experiments performed.
Mode of CTL activation determines quantity of bystander lysis.
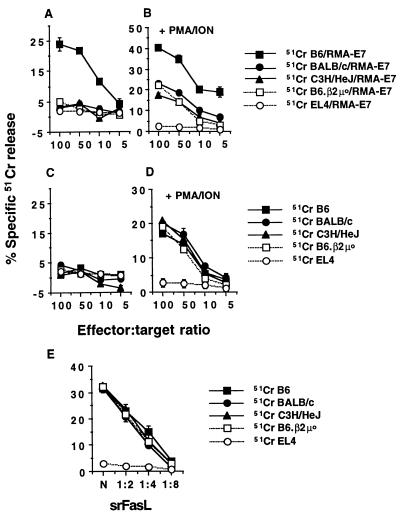
A previous report by Kojima et al. highlighted the fact that maximal bystander lysis was mediated by CTL clones that were stimulated by PMA and ION, thus bypassing TCR stimulation (13). In this scenario, interactions between activated CTL and bystanders could be observed in the absence of third-party, Ag-bearing stimulator cells. These experiments were also of particular interest, since PMA-ION-stimulated CTL of H-2d origin lysed H-2b bystander cells. By contrast, we had previously examined bystander lysis only of syngeneic (H-2b) cells by alloreactive CTL (b anti-k) in the presence of H-2k target cells (34). To further investigate bystander lysis following CTL activation, we stimulated B6.P0 anti-E749-57 CTL with PMA-ION and/or Ag-bearing RMA-E7 cells and evaluated the ability of these CTL to lyse bystander blasts of H-2b, H-2k, and H-2d origin (Fig. 4A to D). B6.P0 anti-E749-57 CTL that were activated with RMA-E7 cells lysed labeled syngeneic bystander B6 blasts but did not significantly lyse H-2k C3H/HeJ blasts, H-2d BALB/c blasts, B6.β2μ0 blasts, or FasL-insensitive EL4 cells (Fig. 4A). None of these bystander cells were lysed by B6.P0 anti-E749-57 CTL in the absence of RMA-E7 cells (Fig. 4C). By contrast, PMA-ION activation of B6.P0 anti-E749-57 CTL stimulated equivalent lysis of all blasts of the three H-2 haplotypes, but not EL4, in the absence of RMA-E7 stimulator cells (Fig. 4D). Only bystander lysis of B6 blasts was greater if RMA-E7 stimulator cells were included in the assay (Fig. 4B). This PMA-ION-activated bystander lysis was also completely inhibited by Fas-Fc fusion protein (data not shown). Importantly, blasts from B6, B6.β2μ0, BALB/c, and C3H/HeJ mice were equally sensitive to srFasL, suggesting that Fas sensitivity did not explain differential susceptibility to B6.P0 anti-E749-57 CTL-mediated bystander lysis (Fig. 4E). It should be noted that preliminary data (not shown) also suggest that alloreactive mouse CTL (b anti-k) do not effectively lyse H-2d BALB/c blasts acting as bystanders. Further experiments with B6 anti-E749-57 CTL were performed, and their mode of bystander lysis was identical to that of B6.P0 anti-E749-57 CTL. In particular, perforin-mediated bystander lysis of allogeneic blasts was not observed, even after pretreatment of B6 anti-E749-57 CTL with PMA-ION (data not shown).
FIG. 4.
Mode of CTL activation determines type and level of bystander lysis. Blasts of B6 (H-2b), B6.β2μ0 (H-2b), C3H/HeJ (H-2k), or BALB/c (H-2d) origin or EL4 cells (H-2b) were 51Cr labeled and used as targets for B6.P0 anti-E749-57 CTL added to Ag-bearing RMA-E7 cells (1:1 ratio) (A), B6.P0 anti-E749-57 CTL prestimulated with PMA-ION (for 3 h) and then added to RMA-E7 cells (1:1 ratio) (B), B6.P0 anti-E749-57 CTL alone (C), and B6.P0 anti-E749-57 CTL prestimulated with PMA-ION for 3 h (D). Lysis by B6.P0 anti-E749-57 CTL was examined in a 4-h 51Cr release assay at the four effector/target ratios illustrated. (E) Lysis of blasts and EL4 target cells by doubling dilutions of srFasL. Cytolysis was expressed as specific 51Cr release after subtraction of spontaneous 51Cr release, which was <15%. These results were calculated as the means ± standard errors of duplicate samples and are representative of two experiments performed.
Bystander lysis by E749-57-specific CTL is not via srFasL.
We considered the possibility that differences in TCR versus PMA-ION stimulation of B6.P0 anti-E749-57 CTL might be due to bystander killing by soluble FasL (sFasL). CTL supernatants were collected after incubation of B6 anti-E749-57 or B6.P0 anti-E749-57 CTL with either RMA-E7 cells or PMA-ION for 3 h. These supernatants were used as media for incubation with 51Cr-labeled syngeneic target cells (Fas sensitive or insensitive). Figure 5 shows that no supernatant had lytic activity toward Fas-sensitive B6 blasts or RMA cells, while sFasL significantly lysed B6 blasts and RMA cells but not EL4 cells. These data indicate that there is no evidence that srFasL is responsible for effects of CTL on bystanders irrespective of the CTL stimulus.
CTL LFA-1–bystander ICAM-1 interaction is critical for bystander lysis.
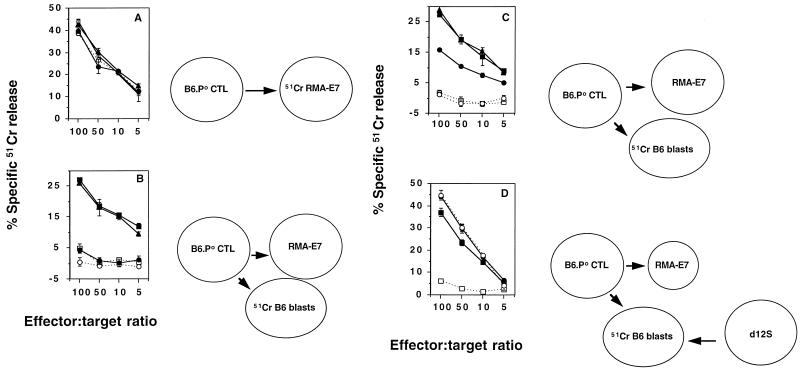
The contact dependence of bystander lysis was investigated with MAbs to surface proteins on the B6.P0 anti-E749-57 CTL. Previous reports have highlighted the importance of an LFA-1–ICAM-1 interaction for Th-1-mediated B-cell apoptosis (40). MAbs were present throughout the assay or were added after 1 h of preactivation of the CTL with RMA-E7 stimulator cells. As previously demonstrated for many forms of Ag-specific lysis (15, 16), the anti-LFA-1 MAb or the anti-CD8 MAb completely inhibited RMA-E7 cell lysis when present throughout an effector-target cell assay (data not shown). By contrast, after 1 h of incubation between B6.P0 anti-E749-57 CTL and 51Cr-labeled RMA-E7 cells, the addition of anti-LFA-1, anti-CD8, or negative control anti-Thy 1.1 MAb did not block E749-57-specific lysis (Fig. 6A). These data suggested that the important interactions involving the TCR-CD8 of CTL and class I molecules-peptide of the RMA-E7 target cells were required for activation of CTL-mediated lysis. Not surprisingly therefore, when present throughout the assay, the anti-LFA-1 and anti-CD8 MAbs could block B6.P0 anti-E749-57 CTL–RMA-E7 interactions, required for TCR-induced FasL expression on CTL, and thus subsequent bystander lysis of labeled B6 blasts in an effector-target-bystander assay (Fig. 6B). However, the simultaneous addition of 51Cr-labeled bystander B6 blasts and MAb to CTL preactivated by RMA-E7 cells 1 h before the assay made it possible to determine the requirements for the delivery of a lethal hit to bystanders by activated B6.P0 anti-E749-57 CTL (Fig. 6C). Anti-LFA-1 MAb completely blocked the lysis of bystander B6 blasts when added 1 h after CTL activation (Fig. 6C). The ability of anti-CD8 MAb to inhibit bystander lysis was considerably diminished when it was added 1 h after the exposure of CTL to RMA-E7 cells (Fig. 6C).
FIG. 6.
CTL LFA-1–bystander ICAM-1 interaction is critical for bystander lysis. (A) Direct lysis. B6.P0 anti-E749-57 CTL and 51Cr-labeled RMA-E7 target cells were coincubated for 4 h (open square). In some tests MAb (anti-LFA-1 [20 μg/ml; open circles], anti-CD8 [20 μg/ml; solid circles], or anti-Thy 1.1 [20 μg/ml; solid triangles]) was added 1 h after the coincubation commenced. (B) Bystander lysis. B6.P0 anti-E749-57 CTL and 51Cr-labeled B6 bystanders were incubated alone (open squares) or together with RMA-E7 target cells in the absence (solid squares) or presence of MAb (symbols as in panel A) for 4 h. (C) Bystander lysis. B6.P0 anti-E749-57 CTL and RMA-E7 stimulator cells were coincubated for 1 h prior to the addition of 51Cr-labeled B6 blasts and MAbs as in panels A and B for a further 4 h. (D) B6.P0 anti-E749-57 CTL and RMA-E7 stimulator cells (squares) or d12S effector cells (circles) were incubated for 4 h with 51Cr-labeled B6 blasts (solid symbols) or B6.ICAM-10 blasts (open symbols). Lysis was determined at the four effector/target ratios illustrated, as described in the legends to Fig. 1 and 2, and results were calculated as the means ± standard errors of duplicate samples.
Lysis of bystander B6 blasts by PMA-ION-preactivated B6.P0 anti-E749-57 CTL was also completely inhibited by anti-LFA-1 MAb but not by anti-CD8 MAb, further confirming a role for LFA-1 in interactions between activated B6.P0 anti-E749-57 CTL and bystander B6 blasts (data not shown). Pretreatment of PMA-ION-preactivated B6.P0 anti-E749-57 CTL with anti-LFA-1 MAb resulted in a complete inhibition of bystander lysis, while pretreatment of bystanders with anti-LFA-1 MAb had no effect (data not shown). Thus, the inhibitory effects of anti-LFA-1 MAb on bystander lysis were not due to the inhibition of CTL–RMA-E7 cell interactions, required for CTL FasL upregulation (38) or LFA-1 activation (7, 21). Only preactivated CTL, as opposed to resting CTL, used their surface LFA-1 molecules to facilitate the effective formation of CTL-bystander conjugates, and after activation, CTL no longer required the signals from Ag-bearing target cells to kill bystander cells. Importantly, experiments with the anti-CD8 MAb demonstrate that the CD8-TCR-dependent activation of CTL is critical for bystander lysis. These data may indicate important changes in the affinity of CTL surface LFA-1 (7, 21) that enabled conjugation with Ag-free bystander cells. Thus, while the expression of FasL and LFA-1 on TCR-activated CTL was necessary, neither alone was sufficient for bystander lysis.
Confirmation that bystander cell ICAM-1 was interacting with CTL LFA-1 was obtained by using 51Cr-labeled blasts from B6.ICAM-10 mice (Fig. 6D). ICAM-1-deficient B6 blasts were not susceptible bystanders, while control ICAM-1-expressing B6 blasts were efficiently lysed by B6.P0 anti-E749-57 CTL. Importantly, B6 and B6.ICAM-10 blasts were equally susceptible to d12S effector cells that kill in a non-MHC-restricted FasL-dependent manner (Fig. 6D). Furthermore, ICAM-1 expression on B6 blasts but not on B6.ICAM0 blasts was confirmed by flow cytometry (data not shown). These results and the data shown in Fig. 6A to C strongly support a critical interaction between activated CTL LFA-1 and bystander ICAM-1. This critical interaction between CTL LFA-1 and bystander ICAM-1 was also observed for B6 anti-E749-57 CTL (data not shown).
Conclusions.
This efficient and rapid lysis of Fas-sensitive bystanders by E749-57 peptide-specific CD8+ CTL extends previously published observations of bystander lysis (2, 6, 13, 34, 41). However, the issue of the variable sensitivity of bystander cells to activated Ag-specific CTL remains somewhat unresolved. In our experimental system we have demonstrated that Ag-specific CTL will lyse syngeneic bystander cells preferentially to third-party allogeneic bystander cells, providing the stimulus is TCR mediated and the bystander expresses class I molecules. If PMA-ION is used to activate Ag-specific CTL they will lyse Fas-sensitive bystanders irrespective of MHC class I molecule expression. Our findings are consistent with an earlier study by Duke that defined self-recognition of bystander target cells by using allospecific CTL (6). However, in contrast, Kojima et al. (13) have shown that allospecific CTL of H-2b origin and specific for H-2k can lyse L1210-Fas bystander cells (H-2d). The discrepancy between the data of Kojima et al. and ours is not simply explained by CTL activation, since these allospecific CTL were not apparently stimulated with PMA-ION (13). Nor is it explained by the lack of ICAM-1 expression on our blast populations, as spleen blasts from B6, BALB/c, and C3H/HeJ mice all expressed similar levels of ICAM-1 (data not shown). The ability of CTL to lyse bystander cells is dependent on important cell-cell interactions and the signals these interactions mediate, and thus the most important interactions between activated CTL and bystander cells of different lineages and activation states may need to be defined to resolve these differences.
In the future, activation of Ag-specific CTL and/or T-cell bystander cells by other T-cell stimuli, such as lectins (e.g., con A), anti-CD2 or anti-CD3 cross-linking, cytokines (e.g., IL-2), or superantigens, will be of interest. Maintenance of clonotype specificity in Fas-mediated apoptosis of mature T cells has been hypothesized to result from important TCR sensitization signals at the time of Fas engagement (9). In these studies, neither resting nor con A-stimulated T-cell blasts acted as bystanders to Ag-induced T-cell death, suggesting that the sensitization event necessary for bystander T-cell death was very specific. By contrast, in our assays, it appeared that the sensitization was provided to bystander T cells after prestimulation with PMA-ION. Arguably, such a powerful stimulus to bystander T cells is not physiologically relevant, but if it is, then these data raise the possibility that T cells of different clonotypes may be destroyed in vigorous or chronic Ag-specific CTL responses. In particular, cytokines such as IL-2 and gamma interferon are important in predisposing T cells to TCR-induced death (4, 17) or creating an environment favorable for Fas-mediated apoptosis (2, 4, 17, 23, 25). Thus, these cytokines may play an important general role in regulating bystander lysis and, consequently, immune responses and the resolution of disease or tumor burden.
The role that CTL-mediated bystander lysis plays in outcomes following virus infection remains to be determined; however, it is becoming evident that many viruses initially enhance the susceptibility of newly infected cells to Fas-mediated apoptosis (31), and thus CTL-mediated bystander lysis of Ag-free infected cells might play an important protective role in this regard. Alternatively, bystander lysis could play a potentially damaging role in chronic infections that involve tissues that can express high levels of Fas (e.g., liver and heart tissues) (2, 24, 29). It is now of paramount importance that bystander lysis be demonstrated convincingly in vivo. In this light, murine models of viral infection or tumor rejection that assess the role of CTL FasL in direct versus bystander cytotoxicity must be developed.
ACKNOWLEDGMENTS
We thank Bruce Loveland for critically reviewing the manuscript. We also thank David Lynch for providing Fas-Fc, TNFR-Fc, and srFasL.
M.J.S. is currently supported by a Wellcome Trust Australasian Senior Research Fellowship and by a Project Grant from the National Health and Medical Research Council of Australia (NH&MRC). R.W.J. is supported by a NH&MRC C. J. Martin Fellowship.
REFERENCES
- 1.Alderson M R, Tough T W, Davis-Smith T, Braddy S, Falk B, Schooley K A, Goodwin R G, Smith C A, Ramsdell F, Lynch D H. Fas ligand mediates activation-induced death in human T lymphocytes. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando K, Hiroishi K, Kaneko T, Moriyama T, Muto Y, Kayagaki N, Yagita H, Okumura K, Imawari M. Perforin, Fas/Fas ligand, and TNF-α pathways as specific and bystander killing mechanisms of hepatitis C virus-specific human CTL. J Immunol. 1997;158:5283–5291. [PubMed] [Google Scholar]
- 3.Conzelmann A, Corthesy P, Cianfriglia M, Silva A, Nabholz M. Hybrids between rat lymphoma and mouse T cells with inducible cytolytic activity. Nature. 1982;298:170–172. doi: 10.1038/298170a0. [DOI] [PubMed] [Google Scholar]
- 4.Critchfield J M, Racke M K, Zuniga-Pflucker J C, Cannella B, Raine C S, Goverman J, Lenardo M J. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science. 1994;263:1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 5.Dhein J, Walczak H, Baumler C, Debatin K-M, Krammer P H. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 6.Duke R C. Self-recognition by T cells. Bystander killing of target cells bearing syngeneic MHC antigens. J Exp Med. 1989;170:59–71. doi: 10.1084/jem.170.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dustin M L, Springer T A. Role of lymphocyte adhesion receptors in transient interactions and cell locomotion. Annu Rev Immunol. 1991;9:27–66. doi: 10.1146/annurev.iy.09.040191.000331. [DOI] [PubMed] [Google Scholar]
- 8.Henkart P A. Mechanism of lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 1985;3:31–58. doi: 10.1146/annurev.iy.03.040185.000335. [DOI] [PubMed] [Google Scholar]
- 9.Hornung F, Zheng L, Lenardo M J. Maintenance of clonotype specificity in CD95/Apo-1/Fas-mediated apoptosis of mature T lymphocytes. J Immunol. 1997;159:3816–3822. [PubMed] [Google Scholar]
- 10.Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 11.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 12.Kent S B H, Hood L E A N. A novel approach to automated peptide synthesis based on new insight into solid phase chemistry. In: Izumiya J, editor. Peptides chemistry. Osaka, Japan: Protein Research Foundation; 1985. p. 217. [Google Scholar]
- 13.Kojima H, Eshima K, Takayama H, Sitkovsky M. Leukocyte function-associated antigen-1-dependent lysis of Fas+ (CD95+/Apo-1+) innocent bystanders by antigen-specific CD8+ CTL. J Immunol. 1997;159:2728–2734. [PubMed] [Google Scholar]
- 14.Kojima H, Shinohara N, Hanaoka S, Someya-Shiorota Y, Takagaki Y, Ohno H, Saitoh T, Katayama T, Yagita H, Okumura K, Shinkai Y, Alt F W, Matsuzawa A, Yonehara S, Takayama H. Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity. 1994;1:357–364. doi: 10.1016/1074-7613(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 15.Lancki D W, Weiss A, Fitch F W. Requirements for triggering of lysis by cytolytic T lymphocyte clones. J Immunol. 1987;138:3646–3653. [PubMed] [Google Scholar]
- 16.Langlet C, Neil G A, Sherman L A. The mechanism of anti-Lyt-2 inhibition of antibody-directed lysis by cytotoxic T lymphocytes. J Immunol. 1987;139:3590–3596. [PubMed] [Google Scholar]
- 17.Lenardo M J. Interleukin-2 programs mouse αβ T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 18.Ljunggren H G, Karre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985;162:1745–1759. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowin B, Beermann F, Schmidt A, Tschopp J. A null mutation in the perforin gene impairs cytolytic T lymphocyte- and natural killer cell-mediated cytotoxicity. Proc Natl Acad Sci USA. 1994;91:11571–11575. doi: 10.1073/pnas.91.24.11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 21.Lub M, van Kooyk Y, Figdor C G. Ins and outs of LFA-1. Immunol Today. 1995;16:479–483. doi: 10.1016/0167-5699(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 22.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 23.Nakamoto Y, Guidotti L G, Pasquetto V, Schreiber R D, Chisari F V. Differential target cell sensitivity to CTL-activated death pathways in hepatitis B virus transgenic mice. J Immunol. 1997;158:5692–5697. [PubMed] [Google Scholar]
- 24.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 25.Ossina N K, Cannas A, Powers V C, Fitzpatrick P A, Knight J D, Gilbert J R, Shekhtman E M, Tomei L D, Umansky S R, Kiefer M C. Interferon-gamma modulates a p53-independent apoptotic pathway and apoptosis-related gene expression. J Biol Chem. 1997;272:16351–16357. doi: 10.1074/jbc.272.26.16351. [DOI] [PubMed] [Google Scholar]
- 26.Podack E R, Hengartner H, Lichtenheld M G. A central role of perforin in cytolysis? Annu Rev Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- 27.Rathmell J C, Cooke M P, Ho W Y, Grein J, Townsend S E, Davis M M, Goodnow C C. CD95 (Fas)-dependent elimination of self-reactive B cells upon interaction with CD4+ T cells. Nature. 1995;376:181–184. doi: 10.1038/376181a0. [DOI] [PubMed] [Google Scholar]
- 28.Rathmell J C, Townsend S E, Xu J C, Flavell R A, Goodnow C C. Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas (CD95)-ligands modulated by the B cell antigen receptor. Cell. 1996;87:319–329. doi: 10.1016/s0092-8674(00)81349-5. [DOI] [PubMed] [Google Scholar]
- 29.Rose N R. Myocarditis—from infection to autoimmunity. Immunologist. 1996;4:67. [Google Scholar]
- 30.Rouvier E, Luciani M-F, Golstein P. Fas involvement in Ca2+-independent T cell-mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sieg S, Huang Y, Kaplan D. Viral regulation of CD95 expression and apoptosis in T lymphocytes. J Immunol. 1997;159:1192–1199. [PubMed] [Google Scholar]
- 32.Sligh J E, Jr, Ballantyne C M, Rich S S, Hawkins H K, Smith C W, Bradley A, Beaudet A L. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1993;90:8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith C A, Farrah T, Goodwin R G. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 34.Smyth M J. Fas ligand-mediated bystander lysis of syngeneic cells in response to an allogeneic stimulus. J Immunol. 1997;158:5765–5772. [PubMed] [Google Scholar]
- 35.Smyth M J, Trapani J A. Granzymes: exogenous proteinases that induce target cell apoptosis. Immunol Today. 1995;16:202–206. doi: 10.1016/0167-5699(95)80122-7. [DOI] [PubMed] [Google Scholar]
- 36.Smyth M J, Sutton V R, Kershaw M H, Trapani J A. Xenospecific cytotoxic T lymphocytes use perforin- and Fas-mediated lytic pathways. Transplantation. 1996;62:1529–1532. doi: 10.1097/00007890-199611270-00030. [DOI] [PubMed] [Google Scholar]
- 37.van den Broek M E, Kagi D, Ossendorp F, Toes R, Vamvakas S, Lutz W K, Melief C J, Zinkernagel R M, Hengartner H. Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vignaux F, Vivier E, Malissen B, Depraetere V, Nagata S, Golstein P. TCR/CD3 coupling to Fas-based cytotoxicity. J Exp Med. 1995;181:781–786. doi: 10.1084/jem.181.2.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walsh C M, Matloubian M, Liu C-C, Ueda R, Kurahara C, Christensen J, Huang M T F, Young J D-E, Ahmed R, Clark W R. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Lenardo M J. Essential lymphocyte function associated 1 (LFA-1): intercellular adhesion molecule interactions for T cell-mediated B cell apoptosis by Fas/APO-1/CD95. J Exp Med. 1997;186:1171–1176. doi: 10.1084/jem.186.7.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang R, Rogers A M, Ratliff T L, Russell J H. CD95-dependent lysis caused by CD4+ T helper 1 effectors. J Immunol. 1996;157:2961–2968. [PubMed] [Google Scholar]
- 42.Zheng L, Fisher G, Miller R E, Peschon J, Lynch D H, Lenardo M J. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 43.Zhu X, Tommasino M, Vousden K, Sadovnikava E, Rappuoli R, Crawford L, Kast M, Melief C J, Beverley P C, Stauss H J. Both immunization with protein and recombinant vaccinia virus can stimulate CTL specific for the E7 protein of human papilloma virus 16 in H-2d mice. Scand J Immunol. 1995;42:557–563. doi: 10.1111/j.1365-3083.1995.tb03696.x. [DOI] [PubMed] [Google Scholar]