Abstract
Anthrax is one of the most important zoonotic diseases which primarily infects herbivores and occasionally humans. The etiological agent is Bacillus anthracis which is a Gram-positive, aerobic, spore-forming, nonmotile, rod-shaped bacillus. The spores are resistant to environmental conditions and remain viable for a long time in contaminated soil, which is the main reservoir for wild and domestic mammals. Infections still occur in low-income countries where they cause suffering and economic hardship. Humans are infected by contact with ill or dead animals, contaminated animal products, directly exposed to the spores in the environment or spores released as a consequence of a bioterrorist event. Three classical clinical forms of the disease, cutaneous, gastrointestinal and inhalation, are seen, all of which can potentially lead to sepsis or meningitis. A new clinical form in drug users has been described recently and named “injectional anthrax” with high mortality (>33%). The symptoms of anthrax in the early stage mimics many diseases and as a consequence it is important to confirm the diagnosis using a bacterial culture or a molecular test. With regards to treatment, human isolates are generally susceptible to most antibiotics with penicillin G and amoxicillin as the first choice, and ciprofloxacin and doxycycline serving as alternatives. A combination of one or more antibiotics is suggested in systemic anthrax. Controlling anthrax in humans depends primarily on effective control of the disease in animals. Spore vaccines are used in veterinary service, and an acellular vaccine is available for humans but its use is limited.
Keywords: anthrax, epidemiology, clinical features, diagnosis, treatment
1. Introduction
Anthrax is an ancient zoonotic disease which primarily infects herbivores with humans occasionally being infected. While naturally occurring, it remains a health problem in low- and middle-income countries; its potential misuse as a biological weapon puts all communities at risk [1,2,3,4,5]. Although the human form of the disease is rarely seen in western countries, human cases have been reported. The most notable being the anthrax postal attack in the United States of America in 2001 [6], and the outbreak of injectional anthrax associated with spore-contaminated heroin in 2009–2010 [7].
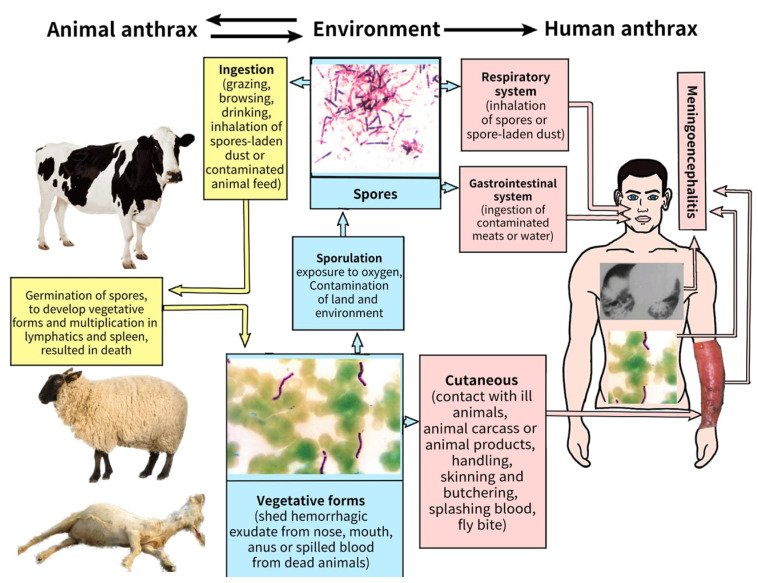
The causative agent of anthrax is a bacteria called Bacillus anthracis, which belongs to the genus Bacillus. The organism infects herbivores through contact with contaminated soils and/or water. Humans become infected through contact with ill or dead animals and their contaminated products. B. anthracis is an aerobic, Gram-positive, spore-forming, non-motile, rod-shaped bacillus. The bacteria are easily grown at 37 °C on blood or nutrient agar. The organism exists in two physical forms, the biologically active vegetative form and the biologically inert spore form. It is the vegetative form which is seen in the tissue of infected individuals and is responsible for the pathology associated with the disease. As the animal succumbs to infection the vegetative form converts into inert, resistant spores which provide a lifeboat for the organism until it is able to infect a new host. The relative resistance of B. anthracis spores to environmental conditions such as drought, heat, rain, cold, radiation and disinfectants is one of the reasons why this organism has been be explored as a potential biowarfare agent [1,8]. Figure 1 summarizes the natural life cycle of B. anthracis.
Figure 1.
The life cycle of Bacillus anthracis in nature. Soil is the main reservoir of the pathogen and is contaminated by spores released from the carcasses of infected animals. Animals grazing on spore-contaminated land become infected resulting in a new cycle of infection, death and release of spores which can potentially contaminate a new location. Wild carnivores and scavenger birds and flies may also contribute to the spread of spores. Humans can be infected by contact with infected animals or contaminated animal products. The figure was created by Fatma Beyzanur Koyuncu, Medical student in Lokman Hekim University, Ankara).
The two principal virulence factors of B. anthracis are tripartite toxin and an antiphagocytic polypeptide capsule, the genes for which are carried on two plasmids designated as pX01 (182 kb) and pX02 (95 kb), respectively. Loss of either of these plasmids reduces the virulence of the organism. The tripartite toxin comprises protective antigen (PA), lethal factor (LF) and edema factor (EF). The role of PA is to transport LF and EF inside target cells where they interact with essential cellular pathways. The toxins are secreted the during multiplication of the vegetative B. anthracis and are responsible for the characteristic symptoms of anthrax [1,8,9].
The aim of this study is to review the current literature on human anthrax and to provide an update on diagnosis and current treatment options. For this purpose, PubMed, Web of Science and Google Scholar were searched using the keywords (alone and in combination) of “anthrax, epidemiology, diagnosis, treatment and therapy” since 2010. Related articles were selected for this review.
2. Epidemiology
Human anthrax is classified under two main headings: agricultural or industrial anthrax. In parts of the world with access to effective healthcare and veterinary services, cases of human infection are rare. Unfortunately, human infection still occurs in low- and middle-income countries where the disease is endemic in livestock and it can be found in the environment. Infection is common in farm animals (cattle, sheep, goats, horses, etc.) and wildlife (elephant, bison, buffalo, zebras, etc.) with occasional outbreaks in humans, with recent cases being reported from most areas of the world excluding northern and central Europe [1,2,5,10,11,12,13,14,15,16,17]. Industrial anthrax is the result of occupational exposure to spore-contaminated animal products such as wool and hair, which accounted for 50% or more of human cases until late in the 20th century [18] (Table 1).
Table 1.
Human anthrax: Common routes of transmission, risk factors and clinical presentations.
| TRANSMISSION OF THE INFECTION | COMMENTS |
|---|---|
| Contact with dying or dead animals | Slaughtering and skinning, handling and processing of dead animals. The disposal of contaminated carcasses |
| Contact with contaminated animal products | Wool coats, shaving brushes, leather (e.g., drumheads made of animal skin) and bone meal (e.g., fertilizer). |
| Ingestion of contaminated meat | Eating contaminated, uncooked, improperly cooked meat, traditional raw food or meat products. |
| Self-injection | Injection of contaminated, illegal heroin |
| Nosocomial transmission | Human to human spread is rare |
| RISK FACTORS | |
| Living in an endemic area | |
| Agricultural occupations | Herdsman, butchers, skinners, slaughterhouse workers, diary workers, veterinarians |
| Industrial occupations | Tanners, leather gift makers, furriers, shoemakers, drum makers, carpet weavers, wool spinners, bone meal processers, wool textile factory workers |
| Illegal drug use | Injection of contaminated materials |
| CLINICAL PRESENTATIONS | COMPLICATIONS * |
| Cutaneous anthrax | Sepsis Meningoencephalitis Pneumonia |
| Gastrointestinal Anthrax Oropharyngeal Intestinal | |
| Inhalation anthrax |
* Sepsis, meningitis or pneumonia may occur due to the pathogen spreading from the primary site of infection.
3. Clinical Presentations
Naturally occurring anthrax is seen in one of three classical clinical forms: cutaneous, gastrointestinal and inhalational. The severity of infection depends on the innate and specific immunity of the patient, virulence and the number of infecting bacteria [1,8,9]. The majority of cases (>95%) are cutaneous in nature, with a mortality rate of less than 3–5% due to the availability of effective antibiotics [8,12,18]. As rare complications, sepsis and meningoencephalitis can develop due to spread from the primary lesion [1,7,8,10,12]. The incidence of the other forms of infection is noted as 12% for inhalation anthrax, 5% for gastrointestinal anthrax and 4% for primary meningitis. Injectional anthrax is a newly described clinical form reported in heroin users as a consequence of injecting spore-contaminated heroin with a mortality of 9–33% [7,18].
3.1. Cutaneous Anthrax
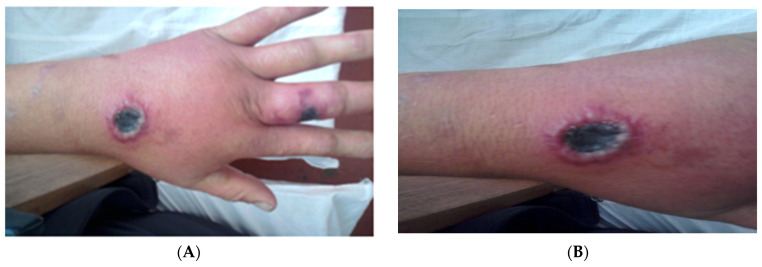
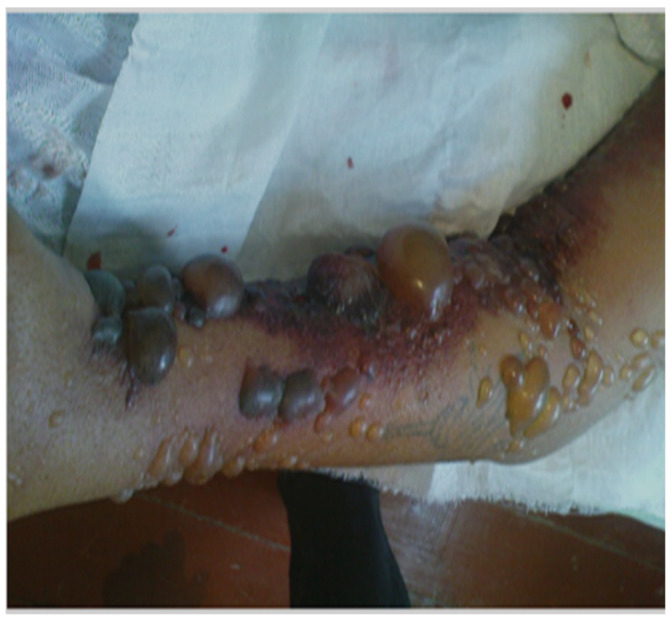
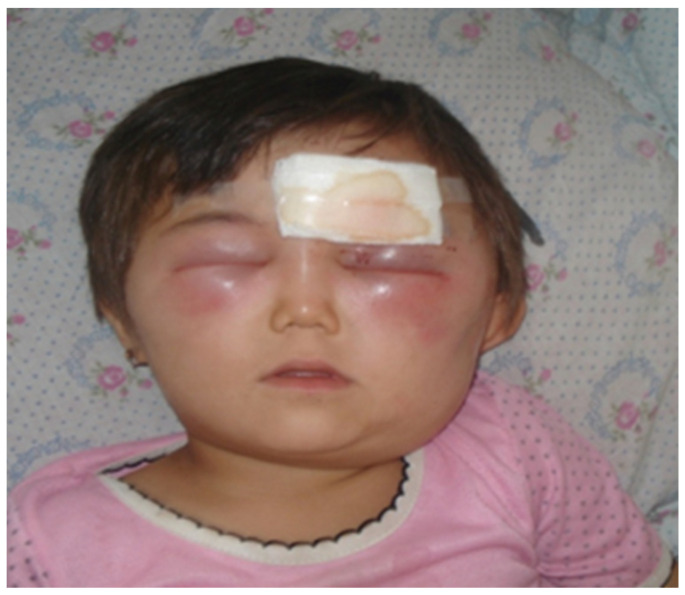
The incubation period is between 2–7 days (range 1–19 days) with the majority of the lesions occurring on exposed areas of the body such as the hands, arms, face and neck. A lesion begins as a pruritic papule and typically progresses to a ring of vesicles surrounded by erythema and edema within 2–4 days (Figure 2). Some lesions may be severe and extended for some distance (Figure 3). Extensive edema and toxemia can be seen in cases where the lesions occur on the face and neck (Figure 4). The formation of the eschar its subsequent resolution can take 2–6 weeks, regardless of treatment [1,8,16,19].
Figure 2.
Typical lesions of cutaneous anthrax on the hand (A) and leg (anterior side) (B). The lesions are characterized by a central eschar with a ring of vesicles and surrounding edema that is characteristically painless. (Images supplied by Professor Ainura Kutmanova, and Dr. Saparbai Zholdoshev).
Figure 3.
Typical appearance of a severe form of cutaneous anthrax lesions on the arm. Extensive erythema and oedema as well as hemorrhagic bullae can be seen (Image supplied by Professor Ainura Kutmanova and Dr. Saparbai Zholdoshev).
Figure 4.
A severe form of cutaneous anthrax on the face. Extensive erythema and oedema can be seen (Image supplied by Professor Ainura Kutmanova and Dr. Saparbai Zholdoshev).
The differential diagnosis of cutaneous anthrax should consider staphylococcal and streptococcal skin and lymph node infections, erysipelas, orf, syphilitic chancre, cutaneous tuberculosis, ecthyma gangrenosum, ulceroglandular tularemia, plague, glanders, rickettsial infection and rat-bite fever [1,8,12,16].
3.2. Gastrointestinal Anthrax
The infection occurs within 3–7 days following the ingestion of B. anthracis within contaminated food or drinks. Lesions can be seen any point along the gastrointestinal tract. Two clinical forms are described in the literature: oropharyngeal and gastrointestinal [1,8,10,18].
The clinical features of oropharyngeal anthrax are fever, sore throat, dysphagia, hoarseness, painful regional lymphadenopathy, soft tissue edema and swelling in the neck. Streptococcal pharyngitis and tonsillitis, parapharyngeal abscess, Vincent angina, Ludwig angina, diphtheria and deep tissue infection as potential causes can be eliminated by the isolation of B. anthracis from the lesions [8,20,21].
The initial symptoms of intestinal anthrax include fever, nausea, vomiting, anorexia and diarrhea. As the infection progresses, symptoms include acute abdominal pain, hematemesis, bloody diarrhea and massive ascites followed by toxemia and shock which results in death. The lesions occur most commonly on the wall of the terminal ileum or cecum. The diagnosis should be confirmed by the isolation of B. anthracis from blood, ascites and the lesions [1,8,22,23].
3.3. Inhalation Anthrax
Although rare, this clinical form is mostly seen as a consequence of industrial exposure or an intentional release. The mortality is recorded at over 80% despite treatment. Following an incubation period of 1–7 days, nonspecific initial symptoms including mild fever, fatigue, malaise, myalgia, nonproductive cough and some chest or abdominal pain are seen. The disease progresses to the severe phase which is characterized by high fever, toxemia, dyspnea and cyanosis. Widening of the mediastinum is described as a typical finding of inhalation anthrax. Pleural effusion and parenchymal infiltrations can also be seen. Hypothermia and shock develop and ultimately result in death. Meningitis may develop as a complication in up to half of patients. Inhalation anthrax mimics community-acquired pneumonia and many diseases involving the pulmonary system [1,8,18].
3.4. Injectional Anthrax
This refers to a new clinical form of anthrax in which soft tissue is infected at the injectional site and leads to toxemia and sepsis. Gas gangrene, necrotizing soft tissue infections and severe cellulitis should be discounted [8,10,18].
4. Diagnosis
The procedures for the diagnosis of anthrax should be as follows: patient’s history, clinical examination for signs and symptoms, routine laboratory examination, radiological examinations and microbiological testing. A history of travel, residence in an endemic region, a job which involves working with animals, exposure to sick or dead animals and the handling of contaminated animal materials could indicate anthrax. Suspected cases should be confirmed by the collection of appropriate samples from the patient’s lesions and subsequent laboratory examination according to the WHO Guidance [1,8,10,16,18]. These samples include swabs from cutaneous lesions, blood, sputum, pulmonary effusion or bronchial biopsy specimens in cases of suspected inhalational anthrax. They also include samples from oropharyngeal lesions, ascites fluid, feces and vomit in suspected cases of intestinal anthrax, and cerebrospinal fluid when meningitis is suspected [1,8].
When biochemical and blood parameters are evaluated, the leukocyte count is usually less than 10 × 103 cells/µL in mild cutaneous cases. In complicated cutaneous infections, toxemic shock, systemic anthrax; leukocytosis with neutrophilia, hypoalbuminemia, hyponatremia, and rising aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels may be detected. If severe sepsis develops, leukopenia, thrombocytopenia and disseminated intravascular coagulation (DIC) may occur [8,24].
The identification of the pathogen is based on a combination of microscopy and culture. The vegetative form of the bacteria appears as a Gram-positive rod-shaped organism. Confirmation of the presence of a capsule which surrounds virulent forms of the bacterium can be confirmed using polychrome methylene blue or Indian ink. Staining with methylene blue reveals the presence of blue-black, square-tipped bacilli surrounded by a pink capsule. The organism can grow on a range of general culture media including blood agar. The BACTEC™ FX40 device, an automated blood culture apparatus, is widely used in microbiology laboratories to recover the pathogen from clinical samples [8,24,25].
For specimens in which the bacteria are likely to present in the company of other micro-organisms, a selective agar is recommended such as Polymyxin-Lysozyme-EDTA-Thallous acetate (PLET) agar. It is based on heart infusion agar supplemented with polymyxin B, lysozyme, ethylenediaminetetraacetic acid (EDTA) and thallus acetate. On this medium the bacterium produces colonies which are white, dome-shaped, round and small. Anthrax Blood Agar (ABA) containing cycloheximide, polymyxin B, trimethoprim and sulfamethoxazole is another selective option. When cultured on this medium, the organism produces white or gray non-hemolytic colonies. R&F Anthrax Chromogenic Agar (ChrA) containing cycloheximide, polymyxin B and X-indoxyl-choline phosphate (X-CP) is another option. The role of X-CP in the medium is to detect the presence the phosphatidylcholine phospholipase C enzyme, which is secreted by B. anthracis, B. cereus and B. thuringiensis. Following 24 h incubation, colonies of B. anthracis appear frosted glass-like in appearance and are cream to faded blue in color, with developing white edges following a further 24 h incubation. Finally, Chromogenic Bacillus Cereus Agar and Cereus Ident Agar can be used as selective media. These media include a chromogenic substrate, 5-bromo-4-chloro-3-indolyl-ß-glucopyranoside, which is degraded by the ß-glucosidase enzyme which is expressed by most species of Bacillus. B. anthracis colonies are a white-creamy color on these media [8,24,26].
B. anthracis can be differentiated from other Bacillus species using a range of simple first-line laboratory tests which include gamma phage susceptibility, catalase production, lack of motility, lack of hemolytic activity when cultured on blood agar and susceptibility to penicillin [24,27]. Although microbiological techniques are the best way to identify the bacteria, they occasional generate ambiguous results, particularly when attempting to differentiate the pathogen from closely related strains of Bacillus cereus. Many phenotypic features of B. anthracis may also be displayed by some B. cereus strains. For example, there have also been reports of strains of B. anthracis that are hemolytic and are resistant to both penicillin and the gamma phage.
To determine the sensitivity of the organism to antibiotics and determine the most appropriate treatment regimen it is necessary to cultivate the agent. The EUCAST (The European Committee on Antimicrobial Susceptibility Testing) has issued guidelines for the rapid antimicrobial susceptibility testing (RAST) of bacteria such as B. anthracis, where rapid results are required to reduce morbidity and mortality [25,28]. Shifman et al. (2021) performed antimicrobial tests (disc diffusion and E-test) directly from blood cultures containing B. anthracis and confirmed the feasibility of this approach [25].
The use of DNA amplification-based PCR (Polymerase Chain Reaction) and Real-time PCR tests can be used for the definitive and rapid diagnosis of B. anthracis in clinical and environmental specimens. Diagnostic targets include specific DNA regions in the following genes: pagA (pXO1), capB (pXO2), capC (pXO2) and Ba813 (chromosomal) [29,30,31,32]. More recently it has been reported that newer isothermal DNA amplification techniques such as RPA (recombinase polymerase amplification), HDA (helicase-dependent amplification) and LAMP (loop-mediated isothermal amplification) can be used. Rapid DNA-based methods are particularly useful for the confirmation of the cause of infection in patients who have been treated with antibiotics which would prevent the bacteria from growing on culture. In addition to DNA-based methods, immunological approaches can also be employed to diagnose the presence of the pathogen. These include flow cytometry analysis using fluorescently labeled antibodies, FRET (Förster resonance energy transfer), ELISA (Enzyme-linked immunosorbent assay), Luminex test, MPFIA (magnetic particle fluorogenic immunoassay) and ABICAP (Antibody Immuno Column for Analytical Processes) immunofiltration. Antibody-based lateral flow devices have been developed for the screening of environmental samples but are not suitable for use with clinical samples [33,34,35].
In recent years there has been a focus on the development of biosensors capable of the rapid and specific detection of B. anthracis. There are currently four types of biosensor platforms: Genosensors (Nucleic Acid Probes), Immunosensors (Antibody Probes), Aptasensors (Aptamers) and Peptide-Nucleic Acid Chimera Probes (PNAs). They employ a range of signal generation approaches which include electrochemical (amperometric, potentiometric and conductometric), optical and piezoelectric. Genosensors work on the principle that the binding of pathogen-specific DNA to a probe generates a signal. To date, genosensors have been developed which target pagA, lef and BA813 [32,36,37,38]. Impedimetric aptasensors have also been used for single-step identification of B. anthracis spore simulants [39]. Antibody probes that recognize B. anthracis-specific antigenic structure have been incorporated into immunosensors such as an ultrasensitive portable capillary biosensor (UPAC) and a magnesium niobate-lead titanate/tin (PMN-PT/Sn) piezoelectric microcantilever sensor (PEMS) [40,41]. Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry (MS) has also been used to detect the pathogen in clinical and environmental specimens [42].
While the detection of an antibody response to the pathogens toxins has little diagnostic value in the early stages of the disease, serum samples should be obtained at 0 to 7 days of illness and at 14 to 28 days to allow the clinical team to confirm diagnosis. There are several rapid anthrax-PA ELISA kits which have been approved by the US FDA for this purpose [24].
5. Management
Anthrax, appearing under the names of “sacred fire” (ignissacer) or “Persian fire” (ignispersicus), has been known since antiquity, and was described in the Murrain of Noricum by the Roman poet Virgil. In 1788, S.S. Andrievsky discovered that anthrax in humans and animals had the same etiology, and F. Brawell proved the possibility of anthrax transmission from animals to humans. A pure culture of the pathogen was obtained by Koch in 1876, and in 1881 Pasteur developed a live vaccine against the disease in animals [43]. In spite of these grand discoveries, the issues of an effective anthrax treatment in humans had yet to be addressed. In the early 1900s, the development of therapeutic sera was one of the most significant practical objectives in various laboratories all over the world. At that time, along with Sclavo’s serum, surgical excision and antiseptic chemicals were concurrently used in the practice. Salvarsan and neoarsphenamine in a combination with Sclavo’s serum were used for anthrax treatment up to the 1940s, when eventually sulfonamides and antibiotics replaced them. Nowadays, antibiotics are considered to be the main therapy for patients with anthrax [44].
Currently, antibiotic therapy is still considered the main option for anthrax treatment. However, the approach to anthrax treatment differs from other bacterial infections due to such features as toxin production, the antibiotic resistance problem and high frequency of meningitis occurrence. In vitro, B. anthracis clinical isolates are susceptible to various antibiotics including penicillin, aminoglycosides, macrolides, quinolones, carbapenems, tetracyclines, vancomycin, clindamycin, rifampicin, cefazolin and linezolid. The recommendations for antibiotic prescription differ depending on the site and severity of the disease [16,45,46]. Thus, in naturally occurring anthrax cases, penicillin G and amoxicillin are the first-choice drugs while doxycycline and ciprofloxacin are the alternative agents, in a treatment scheme with a duration of 5–7 days for mild and uncomplicated cutaneous anthrax cases, and 10–14 days for complicated cutaneous and systemic anthrax cases [12,47,48,49]. In mild and uncomplicated cases with cutaneous anthrax, an oral antibiotic is suggested. In severe cases such as inhalational or gastrointestinal anthrax, meningoencephalitis, sepsis or cutaneous anthrax with extensive edema, antibiotics should be administered intravenously; when the fever has subsided to normal, antibiotic therapy may be switched to oral. In severe cases or internal organ anthrax, initial antibiotic choice should be combined with one or two of the following antibiotics: penicillin, ampicillin, ciprofloxacin, imipenem, meropenem, vancomycin, rifampin (rifampicin), clindamycin, linezolid or aminoglycoside [1,8,10,12].
The recommended antibiotic regimens in adult cases are as follows: procaine penicillin G, 0.6–1.2 M units intramuscularly every 12–24 h; amoxicillin 500 mg orally every 6–8 h; doxycycline 100 mg intravenously or orally every 12 h; ciprofloxacin 200–400 mg intravenously every 12 h, followed by 500–750 mg orally every 12 h [48,49,50]. If such regimens are followed, cutaneous lesions usually become sterile within the first 24 h with edema regressing within 24 to 48 h. However, it should be noted that although due to early treatment the size of the lesion will be limited, the evolutionary stages of the lesion will not be altered. Penicillin G is considered to be the first-choice antibiotic in these cases and should be prescribed at the dosage of 2400 mg (4 M units) every 4–6 h by infusion until the resolution of patient’s symptoms and normalization of the temperature [1,12,48].
Ciprofloxacin and doxycycline are considered to be the first-choice agents in cases of biological weapon or bio-terrorism-related anthrax, and are prescribed in the following regimens: ciprofloxacin 200–400 mg intravenously every 12 h, followed by 500–750 mg orally every 12 h; doxycycline 100 mg intravenously or orally every 12 h with the treatment duration of 42–60 days [47].
5.1. Cutaneous Anthrax
Antibiotic administration is the main treatment for naturally occurring anthrax. In mild or uncomplicated cases, monotherapy with intramuscular penicillin G or the oral antibiotics doxycycline or ciprofloxacin are effective. In severe and complicated cases antibiotics should be administered intravenously. During the acute inflammatory period cutaneous lesions should be dressed and covered with a sterile wrap; surgical intervention should be avoided as it can lead to dissemination and a poor outcome [1,50]. However, the surgical debridement of infected soft tissue in combination with antibiotics and supportive therapy may be life-saving in cases of injectional anthrax [7,8,10,18].
In cases where the head and neck are affected, adjunctive corticosteroids may be prescribed as an anti-edema treatment to avoid possible serious complications. Furthermore, careful monitoring for airway compromise should be carried out as respiratory support for airway edema may be required [1,8,51].
5.2. Gastrointestinal Anthrax
As clinical manifestations of gastrointestinal anthrax vary largely, imitating a range of different diseases, physicians of the endemic areas should be especially aware of this clinical form. Due to the lack of awareness, patients undergo unnecessary interventions such as abdominal surgery because of acute abdomen syndrome or, on the contrary, fail to receive adequate medical treatment if only mild diarrhea is present. In case of gastrointestinal anthrax, initial antibiotic therapy is suggested, using a combination of penicillin G with streptomycin or other aminoglycosides. To improve the outcomes of patients diagnosed with gastrointestinal anthrax, the treatment should be followed as [1]: a. Give an appropriate antibiotic in adequate dose intravenously. b. Careful monitore fluid, electrolyte and protein losses and in-time replacement for patient. c. To consider early surgical resection of the necrotic intestine. Surgical treatment in the form of wide resection of the infected and necrotic parts of the intestine with primary anastomosis may be lifesaving.
5.3. Inhalation Anthrax
In case of inhalation anthrax, penicillin G may be used in the combination with clindamycin or clarithromycin initially. In case of allergy to penicillin, other alternative regimens can be used in the therapy.
In inhalation anthrax, patients may require mechanical ventilation due to respiratory failure caused by reaccumulating pleural effusions. However, the necessity and duration of ventilation may be reduced by performing pleural space drainage [51]. According to Holty, J.E. et al., this procedure improved outcomes in a series of cases by decreasing the level of lethal factors and mechanical lung compression [52,53]. Thus, performing an aggressive pleural fluid drainage at the earliest possible time after the detection of any clinically or radiographically apparent pleural effusions is highly recommended. It should be noted that chest tube drainage is more preferred compared to thoracentesis as prolonged drainage may be required. In order to remove gelatinous or loculated collections, thoracotomy or video-assisted thoracic surgery is recommended [51].
5.4. Anthrax Meningoencephalitis
A combination of antibiotics is preferred with one of them being able to penetrate to the central nervous system. If anthrax meningoencephalitis is suspected, empiric treatment should include three or more antimicrobial drugs showing an adequate level of CNS penetration with one of them having bactericidal activity, and another one should be a protein synthesis inhibitor. The duration of treatment for anthrax meningoencephalitis should be carried out for at least two weeks or until the clinical stability of the patient’s condition. Considering the high frequency of fatal outcomes, some experts recommend to provide at least a three-week treatment in cases when the diagnosis of meningitis cannot be excluded. An algorithm is suggested by the experts for the diagnosis of meningitis [51]. Due to the good CNS penetration and relatively low level of antimicrobial resistance, ciprofloxacin (400 mg every 8 h) is recommended as a component with bactericidal activity in the treatment scheme of anthrax meningoencephalitis, while levofloxacin (750 mg every 24 h) and moxifloxacin (400 mg every 24 h) are alternatives [51,54]. A combination of penicillin G or a fluoroquinolone with rifampicin is currently considered the first-choice treatment in such cases due to its effective activity against B. anthracis and fast penetration into the cerebrospinal fluid. The recommended dose of crystalline penicillin G is 20–24 million units per day divided for intravenous administration every 2–4 h, and rifampicin should be prescribed in a dose of 600–1200 mg per day (can be administered intravenously or via an enteral tube if needed) [1,12]. Due to the good CNS penetration and relatively low level of antimicrobial resistance, ciprofloxacin (400 mg every 8 h) is recommended as it has bactericidal activity, with levofloxacin (750 mg every 24 h) and moxifloxacin (400 mg every 24 h) as alternatives if required [51,54].
Supportive therapy plays an important role in anthrax meningoencephalitis as respiratory support and anti-edema therapy for the brain may be life-saving measures in most cases. In order to provide supportive therapy, such measures as early initiated assisted respiration, maintaining of fluid and electrolyte balance and anti-edema treatment (100 mL of 20% mannitol intravenously every 8 h and 100 mg of hydrocortisone every 6 h) are essential [1].
In addition to antibiotic therapy, specific antitoxin serum for anthrax may be used in injectional anthrax and systemic anthrax. A polyclonal anthrax immune globulin intravenous (AIGIV-Anthrivig) drug and two monoclonal antitoxins, raxibacumab and obiltoxaximab, were developed in the USA and have shown beneficial effects in an animal model [55].
Vaccines to project humans against anthrax are available. Attenuated live spore vaccines are available in China and Russia to protect at-risk individuals. Due to concerns over the reactogenicity of spore-based vaccines in humans, acellular vaccines have been developed in the UK and the USA. Protective antigen is the principal protective immunogen in these licensed human vaccines [56,57,58,59]. Both require multiple doses to induce protection, and because of the manner by which they were developed, they are relatively crude products containing trace amounts of LF, EF and other bacterial antigens [60]. While primarily intended to provide protection against infection, they have a role to play in the treatment of individuals who have inhaled spores of the pathogen. The US CDC recommends a post-exposure regimen of 60 days of appropriate antimicrobial prophylaxis combined with 3 subcutaneous doses of the anthrax vaccine [61]. The reasons for this are because the spore form of the pathogen, which is resistant to antibiotics, could remain dormant in the lungs until antibiotic treatment has stopped, at which time the spore could germinate to initiate a new infection. The administration of the vaccine allows the individual to develop a protective immune response which is able to deal with any germinating bacteria.
6. Conclusions
Anthrax is still an endemic disease in middle- and low-income agricultural countries. The main transmission of anthrax to human is by contact with ill or dead animals and contaminated animal products. The majority of clinical forms is cutaneous anthrax. Suspected cases should be confirmed by laboratory tests. Human isolates are generally in vitro susceptible to many antibiotics. Penicillin G, amoxicillin, ciprofloxacin and doxycycline are widely using in the clinical practice of naturally occurring anthrax. The prevention of human anthrax is based on the control of animal infection, education of animal owners and occupational risk groups. For example, ill or dead animals ought not be slaughtered, skinned or butchered for consumption of their meats or have their products used. The control of infections in animals consists of close surveillance, vaccination of animals against anthrax and good veterinary practices, which include the burying or cremation of infected animal carcasses and the use of effective decontamination and disinfection procedures. For human immunization, an acellular vaccine is only available in the UK and the USA, and it is used mostly in occupational and military settings. A live spore vaccine for immunization of human has been produced and used in China and Russia.
Author Contributions
Conceptualization, M.D., G.D. and A.K.; methodology, M.D., G.D. and A.K. software, M.D., G.D. and A.K.; validation, M.D., G.D. and A.K.; formal analysis, M.D., G.D. and A.K. investigation, M.D., G.D. and A.K.; resources, M.D., G.D. and A.K.; writing—original draft preparation, M.D., G.D., A.K. and L.B.; writing—review and editing, M.D., G.D., A.K. and L.B.; visualization, M.D., G.D., A.K. and L.B.; supervision, M.D. and L.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.WHO. OIE. FAO . Anthrax in Humans and Animals. 4th ed. World Health Organization; Geneva, Switzerland: 2008. [Google Scholar]
- 2.Bakhteeva I., Timofeev V. Some peculiarities of Anthrax epidemiology in herbivorous and carnivorous animals. Life. 2022;10:870. doi: 10.3390/life12060870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pittiglio C., Shadomy S., El Idrissi A., Soumare B., Lubroth J., Makonnen Y. Seasonality and Ecological Suitability Modelling for Anthrax (Bacillus anthracis) in Western Africa. Animals. 2022;12:1146. doi: 10.3390/ani12091146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eremenko E., Pechkovskii G., Pisarenko S., Ryazanova A., Kovalev D., Semenova O., Aksenova L., Timchenko L., Golovinskaya T., Bobrisheva O., et al. Phylogenetics of Bacillus anthracis isolates from Russia and bordering countries. Infect. Genet. Evol. 2021;92:104890. doi: 10.1016/j.meegid.2021.104890. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W.Y., Wang L.Y., Zhang X.S., Han Z.H., Hu W.B., Qian Q., Haque U., Soares Magalhaes R.J., Li S.L., Tong S.L., et al. Spatiotemporal clustering analysis and risk assessments of human cutaneous Anthrax in China, 2005–2012. PLoS ONE. 2015;10:e0133736. doi: 10.1371/journal.pone.0133736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jernigan D.B., Raghunathan P.L., Bell B.P., Brechner R., Bresnitz E.A., Butler J.C., Cetron M., Cohen M., Doyle T., Fischer M., et al. Investigation of bioterrorism-related Anthrax, United States, 2001: Epidemiologic findings. Emerg. Infect. Dis. 2002;8:1019–1028. doi: 10.3201/eid0810.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Booth M., Donaldson L., Cui X., Sun J., Cole S., Dailsey S., Hart A., Johns N., McConnell P., McLennan T., et al. Confirmed Bacillus anthracis infection among persons who inject drugs, Scotland, 2009–2010. Emerg. Infect. Dis. 2014;20:1452–1463. doi: 10.3201/eid2009.131481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doganay M. Anthrax. In: Cohen J., Powderly W.G., Opal S.M., editors. Infectious Diseases. 4th ed. Elsevier; Amsterdam, The Netherlands: 2017. pp. 1123–1128. [Google Scholar]
- 9.Moayeri M., Leppla S.H., Vrentas C., Pomerantsev A.P., Liu S. Anthrax pathogenesis. Annu. Rev. Microbiol. 2015;69:185–208. doi: 10.1146/annurev-micro-091014-104523. [DOI] [PubMed] [Google Scholar]
- 10.Doganay M., Demiraslan H. Human anthrax as a re-emerging disease. Recent Pat. Antiinfect. Drug Discov. 2015;10:10–29. doi: 10.2174/1574891X10666150408162354. [DOI] [PubMed] [Google Scholar]
- 11.Badri R., Uwishema O., Wellington J., Thambi V.D., Pradhan A.U., Adanur I., Onyeaka C.V.P., Onyeaka H. Anthrax outbreak amidst the COVID-19 pandemic in Africa: Challenges and possible solutions. Ann. Med. Surg. 2022;81:104418. doi: 10.1016/j.amsu.2022.104418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doganay M., Metan G. Human anthrax in Turkey from 1990 to 2007. Vector-Borne Zoonotic Dis. 2009;9:131–140. doi: 10.1089/vbz.2008.0032. [DOI] [PubMed] [Google Scholar]
- 13.Galante D., Manzulli V., Serrecchia L., Taranto P.D., Hugh-Jones M., Hossain M.J., Rondinone V., Cipolletta D., Pace L., Iatarola M., et al. Investigation on Anthrax in Bangladesh during the outbreaks of 2011 and definition of the epidemiological correlations. Pathogens. 2021;10:481. doi: 10.3390/pathogens10040481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alam M.E., Kamal M.M., Rahman M., Kabir A., Islam M.S., Hassan J. Review of anthrax: A disease of farm animals. J. Adv. Vet. Anim. Res. 2022;9:323–334. doi: 10.5455/javar.2022.i599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kracalik I.T., Malania L., Tsertsvadze N., Manvelyan J., Bakanidze L., Imnadze P., Tsanava S., Blackburn J.K. Evidence of local persistence of human Anthrax in the country of Georgia associated with environmental and anthropogenic factors. PLoS Negl. Trop. Dis. 2013;7:e2388. doi: 10.1371/journal.pntd.0002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kutmanova A., Doganay M., Zholdoshev S. Human anthrax in Kyrgyz Republic: Epidemiology and clinical features. J. Infect. Public Health. 2020;13:1161–1165. doi: 10.1016/j.jiph.2020.02.043. [DOI] [PubMed] [Google Scholar]
- 17.Hodnik J.J., Acinger-Rogić Ž., Alishani M., Autio T., Balseiro A., Berezowski J. Overview of cattle diseases listed under category C, D or E in the Animal Health Law for which control programmes are in place within Europe. Front. Vet. Sci. 2021;8:688078. doi: 10.3389/fvets.2021.688078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendricks K., Person M.K., Bradley J.S., Mongkolrattanothai T., Hupert N., Eichacker P., Friedlander A.M., Bower W.A. Clinical features of patients hospitalized for all routes of Anthrax, 1880–2018: A Systematic Review. Clin. Infect. Dis. 2022;75((Suppl. 3)):S341–S353. doi: 10.1093/cid/ciac534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parlak E., Parlak M. Human cutaneous anthrax, the east anatolian region of Turkey 2008–2014. Vector-Borne Zoonotic Dis. 2016;16:42–47. doi: 10.1089/vbz.2015.1835. [DOI] [PubMed] [Google Scholar]
- 20.Doganay M., Almac A., Hanagasi R. Primary throat anthrax. A report of six cases. Scand. J. Infect. Dis. 1986;18:415–419. doi: 10.3109/00365548609032357. [DOI] [PubMed] [Google Scholar]
- 21.Sirisanthana T., Brown A.E. Anthrax of the gastrointestinal tract. Emerg. Infect. Dis. 2002;8:649–651. doi: 10.3201/eid0807.020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanafani Z.A., Ghossain A., Sharara A.I., Hatem J.M., Kanj S.S. Endemic gastrointestinal anthrax in 1960s Lebanon: Clinical manifestations and surgical findings. Emerg. Infect. Dis. 2003;9:520–525. doi: 10.3201/eid0905.020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meric M., Willke A., Muezzinoglu B., Karadenizli A., Hosten T. A case of pneumonia caused by Bacillus anthracis secondary to gastrointestinal anthrax. Int. J. Infect. Dis. 2009;13:e456–e458. doi: 10.1016/j.ijid.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Martin G.J., Friedlander A.M. Bacillus anthracis. In: Bennett J.E., Dolin R., Blaser M.J., editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. Elsevier; Amsterdam, The Netherlands: 2020. pp. 2550–2569. [Google Scholar]
- 25.Shifman O., Aminov T., Aftalion M., Gur D., Cohen H., Bar-David E., Cohen O., Mamroud E., Levy H., Aloni-Grinstein R., et al. Evaluation of the European Committee on Antimicrobial Susceptibility Testing Guidelines for rapid antimicrobial susceptibility testing of Bacillus anthracis, Yersinia pestis and Francisella tularensis positive blood cultures. Microorganisms. 2021;9:1055. doi: 10.3390/microorganisms9051055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomaso H., Bartling C., Al Dahouk S., Hagen R.M., Scholz H.C., Beyer W., Neubauer H. Growth characteristics of Bacillus anthracis compared to other Bacillus spp. on the selective nutrient media Anthrax Blood Agar and Cereus Ident Agar. Syst. Appl. Microbiol. 2006;29:24–28. doi: 10.1016/j.syapm.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Abshire T.G., Brown J.E., Ezzell J.W. Production and validation of the use of gamma phage for identification of Bacillus anthracis. J. Clin. Microbiol. 2005;43:4780–4788. doi: 10.1128/JCM.43.9.4780-4788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.EUCAST Rapid AST Directly from Blood Culture Bottles. [(accessed on 31 January 2023)]. Available online: https://www.eucast.org/rapid_ast_in_blood_cultures/
- 29.Zasada A.A., Zacharczuk K., Formi’nska K., Wiatrzyk A., Ziółkowski R., Malinowska E. Isothermal DNA amplification combined with lateral flow dipsticks for detection of biothreat agents. Anal. Biochem. 2018;560:60–66. doi: 10.1016/j.ab.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Bentahir M., Ambroise J., Delcorps C., Pilo P., Gala J.L. Sensitive and specific recombinase polymerase amplification assays for fast screening, detection, and identification of Bacillus anthracis in a field setting. Appl. Environ. Microbiol. 2018;84:e00506–e00518. doi: 10.1128/AEM.00506-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamborrini M., Holzer M., Seeberger P.H., Schürch N., Pluschke G. Anthrax spore detection by a luminex assay based on monoclonal antibodies that recognize anthrose-containing oligosaccharides. Clin. Vaccine Immunol. 2010;17:1446–1451. doi: 10.1128/CVI.00205-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zasada A.A. Detection and Identification of Bacillus anthracis: From Conventional to Molecular Microbiology Methods. Microorganisms. 2020;16:125. doi: 10.3390/microorganisms8010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen N., Zahavy E., Zichel R., Fisher M. An internal standard approach for homogeneous TR-FRET immunoassays facilitates the detection of bacteria, biomarkers, and toxins in complex matrices. Anal. Bioanal. Chem. 2016;408:5179–5188. doi: 10.1007/s00216-016-9602-0. [DOI] [PubMed] [Google Scholar]
- 34.Zasada A.A., Formi´nska K., Zacharczuk K., Jacob D., Grunow R. Comparison of eleven commercially available rapid tests for detection of Bacillus anthracis, Francisella tularensis and Yersinia pestis. Lett. Appl. Microbiol. 2015;60:409–413. doi: 10.1111/lam.12392. [DOI] [PubMed] [Google Scholar]
- 35.Kim J., Gedi V., Lee S.C., Cho J.H., Moon J.Y., Yoon M.Y. Advances in anthrax detection: Overview of bioprobes and biosensors. Appl. Biochem. Biotechnol. 2015;176:957–977. doi: 10.1007/s12010-015-1625-z. [DOI] [PubMed] [Google Scholar]
- 36.Raveendran M., Andrade A.F.B., Gonzalez-Rodriguez J. Selective and sensitive electrochemical DNA biosensor for the detection of Bacillus anthracis. Int. J. Electrochem. Sci. 2016;11:763–776. [Google Scholar]
- 37.Ziółkowski R., Oszwałdowski S., Zacharczuk K., Zasada A.A., Malinowska E. Electrochemical detection of Bacillus anthracis protective antigen gene using DNA biosensor based on stem−loop probe. J. Electrochem. Soc. 2018;165:B187–B195. doi: 10.1149/2.0551805jes. [DOI] [Google Scholar]
- 38.Zhang B., Dallo S., Peterson R., Hussain S., Weitao T., Ye J.Y. Detection of anthrax lef with DNA-based photonic crystal sensors. J. Biomed. Opt. 2011;16:127006. doi: 10.1117/1.3662460. [DOI] [PubMed] [Google Scholar]
- 39.Mazzaracchio V., Neagu D., Porchetta A., Marcoccio E., Pomponi A., Faggioni G., D’Amore N., Notargiacomo A., Pea M., Moscone D., et al. A label-free impedimetric aptasensor for the detection of Bacillus anthracis spore simulant. Biosens. Bioelectron. 2019;126:640–646. doi: 10.1016/j.bios.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 40.Mwilu S.K., Aluoch A.O., Miller S., Wong P., Sadik O.A., Fatah A.A., Arcilesi R.D. Identification and quantitation of Bacillus globigii using metal enhanced electrochemical detection and capillary biosensor. Anal.Chem. 2009;81:7561–7570. doi: 10.1021/ac900834e. [DOI] [PubMed] [Google Scholar]
- 41.Waller D.F., Hew B.E., Holdaway C., Jen M., Peckham G.D. Rapid detection of Bacillus anthracis spores using immunomagnetic separation and amperometry. Biosensors. 2016;6:61. doi: 10.3390/bios6040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pauker V.I., Thoma B.R., Grass G., Bleichert P., Hanczaruk M., Zöller L., Zange S. Improved discrimination of Bacillus anthracis from closely related species in the Bacillus cereus Sensu Lato group based on Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2018;56:e01900–e01917. doi: 10.1128/JCM.01900-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sternbach G. The history of anthrax. J. Emerg. Med. 2003;24:463–467. doi: 10.1016/S0736-4679(03)00079-9. [DOI] [PubMed] [Google Scholar]
- 44.James F.S. ‘A remedy for this dread disease’: Achille Sclavo, anthrax and serum therapy in early twentieth-century Britain. Br. J. Hist. Sci. 2022;55:207–226. doi: 10.1017/S0007087422000012. [DOI] [PubMed] [Google Scholar]
- 45.Metan G., Doganay M. The antimicrobial susceptibility of Bacillus anthracis isolated from human cases: A review of Turkish literature. Turk. Klin. J. Med. Sci. 2009;29:229–235. [Google Scholar]
- 46.Doganay M. Ingestional (Oral Route/Enteric) Anthrax: Is It a Problem in Turkey? FLORA. 2009;14:97–104. [Google Scholar]
- 47.Savransky V., Ionin B., Reece J. Current Status and Trends in Prophylaxis and Management of Anthrax Disease. Pathogens. 2020;9:370. doi: 10.3390/pathogens9050370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elbahr U.S., Tekin R., Papić M., Pandak N., Erdem H., Can F.K., Alpat S.N., Pekok A.U., Pehlivanoglu F., Karamese M., et al. Factors leading to dissemination of cutaneous anthrax: An international ID-IRI study. New Microbes New Infect. 2022;48:101028. doi: 10.1016/j.nmni.2022.101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Denk A., Tartar A.S., Ozden M., Demir B., Akbulut A. Cutaneous anthrax: Evaluation of 28 cases in the Eastern Anatolian region of Turkey. Cutan. Ocul. Toxicol. 2016;35:177–180. doi: 10.3109/15569527.2015.1067818. [DOI] [PubMed] [Google Scholar]
- 50.Kutmanova A., Zholdoshev S., Roguski K.M., Sholpanbay uulu M., Person M.K., Cook R., Bugrysheva J., Nadol P., Buranchieva A., Imanbaeva L., et al. Risk Factors for Severe Cutaneous Anthrax in a Retrospective Case Series and Use of a Clinical Algorithm to Identify Likely Meningitis and Evaluate Treatment Outcomes, Kyrgyz Republic, 2005–2012. Clin. Infect. Dis. 2022;75:478–486. doi: 10.1093/cid/ciac537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hendricks K.A., Wright M.E., Shadomy S.V., Bradley J.S., Morrow M.G., Pavia A.T., Rubinstein E., Holty J.E., Messonnier N.E., Smith T.L., et al. Centers for Disease Control and Prevention Expert Panel Meetings on Prevention and Treatment of Anthrax in Adults. Emerg. Infect. Dis. 2014;20:e130687. doi: 10.3201/eid2002.130687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holty J.E., Bravata D.M., Liu H., Olshen R.A., McDonald K.M., Owens D.K. Systematic review: A century of inhalational anthrax cases from 1900 to 2005. Ann. Intern. Med. 2006;144:270–280. doi: 10.7326/0003-4819-144-4-200602210-00009. [DOI] [PubMed] [Google Scholar]
- 53.Walsh J.J., Pesik N., Quinn C.P., Urdaneta V., Dykewicz C.A., Boyer A.E., Guarner J., Wilkins P., Norville K.J., Barr J.R., et al. A case of naturally acquired inhalation anthrax: Clinical care and analyses of anti-protective antigen immunoglobulin G and lethal factor. Clin. Infect. Dis. 2007;44:968–971. doi: 10.1086/512372. [DOI] [PubMed] [Google Scholar]
- 54.Sejvar J.J., Tenover F.C., Stephens D.S. Management of anthrax meningitis. Lancet Infect. Dis. 2005;5:287–295. doi: 10.1016/S1473-3099(05)70113-4. [DOI] [PubMed] [Google Scholar]
- 55.Slay R.M., Cook R., Hendricks K., Boucher D., Merchlinsky M. Pre- and Postlicensure Animal Efficacy Studies Comparing Anthrax Antitoxins. Clin. Infect. Dis. 2022;75((Suppl. 3)):S441–S450. doi: 10.1093/cid/ciac593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fan Y., Moon J.J. Particulate delivery systems for vaccination against bioterrorism agents and emerging infectious pathogens. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017;9:e1403. doi: 10.1002/wnan.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brenneman K.E., Doganay M., Akmal A., Goldman S., Galloway D.R., Mateczun A.J., Baillie L.W. The early humoral immune response to Bacillus anthracis toxins in patients infected with cutaneous anthrax. FEMS Immunol. Med. Microbiol. 2011;62:164–172. doi: 10.1111/j.1574-695X.2011.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galloway D.R., Baillie L. DNA vaccines against anthrax. Expert Opin. Biol. Ther. 2004;4:1661–1667. doi: 10.1517/14712598.4.10.1661. [DOI] [PubMed] [Google Scholar]
- 59.Clark A., Wolfe D.N. Current State of Anthrax Vaccines and Key R&D Gaps Moving Forward. Microorganisms. 2020;8:651. doi: 10.3390/microorganisms8050651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hepburn M.J., Dyson E.H., Simpson A.J., Brenneman K.E., Bailey N., Wilkinson L., Hornby R., Mateczun A.J., Bell M.G., Baillie L.W. Immune response to two different dosing schedules of the anthrax vaccine precipitated (AVP) vaccine. Vaccine. 2007;25:6089–6097. doi: 10.1016/j.vaccine.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 61.CDC Anthrax Vaccine Recommendations. [(accessed on 16 February 2023)]; Available online: https://www.cdc.gov/vaccines/vpd/anthrax/hcp/recommendations.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.