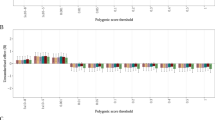
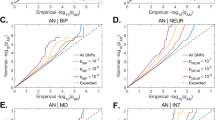
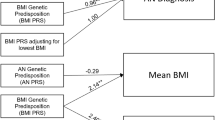
Abstract
Binge eating disorder (BED) is the most common eating disorder, yet its genetic architecture remains largely unknown. Studying BED is challenging because it is often comorbid with obesity, a common and highly polygenic trait, and it is underdiagnosed in biobank data sets. To address this limitation, we apply a supervised machine-learning approach (using 822 cases of individuals diagnosed with BED) to estimate the probability of each individual having BED based on electronic medical records from the Million Veteran Program. We perform a genome-wide association study of individuals of African (n = 77,574) and European (n = 285,138) ancestry while controlling for body mass index to identify three independent loci near the HFE, MCHR2 and LRP11 genes and suggest APOE as a risk gene for BED. We identify shared heritability between BED and several neuropsychiatric traits, and implicate iron metabolism in the pathophysiology of BED. Overall, our findings provide insights into the genetics underlying BED and suggest directions for future translational research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
BED GWAS summary statistics from the MVP data are available on dbGaP (accession no. phs001672). For the external validation sets for the partitioned heritability analysis, we used open chromatin regions from a murine erythroid cell beta-estradiol stimulation model (GEO accession no. GSE114996), open chromatin atlas of adult human brains (GEO accession no. GSE147672) and open chromatin atlas of developing human organs (https://descartes.brotmanbaty.org/bbi/human-chromatin-during-development). For GWAS summary statistics for genetic correlation analyses, see the IEU Open GWAS Project (https://gwas.mrcieu.ac.uk).
Code availability
Software used in this study included the following programs: EIGENSOFT v.6 (https://github.com/dreichlab/eig); FUMA v.1.3.7 (https://fuma.ctglab.nl); GCTA v.1.93.2 (https://yanglab.westlake.edu.cn/software/gcta/#Overview); KING v.2.0 (https://www.chen.kingrelatedness.com); LD score regression v.1.0.1 (https://github.com/bulik/ldsc); liftOver v.1.2.0 (https://genome.ucsc.edu/cgi-bin/hgLiftOver); Minimac v.3 (https://genome.sph.umich.edu/wiki/Minimac3); Multi-ancestry meta-analysis (https://github.com/JonJala/mama); PheMED (https://github.com/DiseaseNeuroGenomics/PheMED); PRS-CS (https://github.com/getian107/PRScs); SuSiE as implemented in echolocatoR72 (https://github.com/RajLabMSSM/echolocatoR).
References
Mitchell, K. S. et al. Binge eating disorder: a symptom-level investigation of genetic and environmental influences on liability. Psychol. Med. 40, 1899–1906 (2010).
Reichborn-Kjennerud, T., Bulik, C. M., Tambs, K. & Harris, J. R. Genetic and environmental influences on binge eating in the absence of compensatory behaviors: a population-based twin study. Int. J. Eat. Disord. 36, 307–314 (2004).
Udo, T. & Grilo, C. M. Prevalence and correlates of DSM-5-defined eating disorders in a nationally representative sample of U.S. adults. Biol. Psychiatry 84, 345–354 (2018).
Brownley, K. A. et al. Binge-eating disorder in adults: a systematic review and meta-analysis. Ann. Intern. Med. 165, 409–420 (2016).
Wonderlich, S. A., Gordon, K. H., Mitchell, J. E., Crosby, R. D. & Engel, S. G. The validity and clinical utility of binge eating disorder. Int. J. Eat. Disord. 42, 687–705 (2009).
Bulik, C. M. et al. The binge eating genetics initiative (BEGIN): study protocol. BMC Psychiatry 20, 307 (2020).
Javaras, K. N. et al. Co-occurrence of binge eating disorder with psychiatric and medical disorders. J. Clin. Psychiatry 69, 266–273 (2008).
Javaras, K. N. et al. Familiality and heritability of binge eating disorder: results of a case-control family study and a twin study. Int. J. Eat. Disord. 41, 174–179 (2008).
Hübel, C. et al. One size does not fit all. Genomics differentiates among anorexia nervosa, bulimia nervosa, and binge-eating disorder. Int. J. Eat. Disord. 54, 785–793 (2021).
Guss, J. L., Kissileff, H. R., Devlin, M. J., Zimmerli, E. & Walsh, B. T. Binge size increases with body mass index in women with binge-eating disorder. Obes. Res. 10, 1021–1029 (2002).
Anderson, D. A., Williamson, D. A., Johnson, W. G. & Grieve, C. O. Validity of test meals for determining binge eating. Eat. Behav. 2, 105–112 (2001).
Kenardy, J. et al. Disordered eating behaviours in women with type 2 diabetes mellitus. Eat. Behav. 2, 183–192 (2001).
Hudson, J. I. et al. Longitudinal study of the diagnosis of components of the metabolic syndrome in individuals with binge-eating disorder. Am. J. Clin. Nutr. 91, 1568–1573 (2010).
Hilbert, A. et al. Meta-analysis on the long-term effectiveness of psychological and medical treatments for binge-eating disorder. Int. J. Eat. Disord. 53, 1353–1376 (2020).
Peat, C. M. et al. Comparative effectiveness of treatments for binge-eating disorder: systematic review and network meta-analysis. Eur. Eat. Disord. Rev. 25, 317–328 (2017).
Gaziano, J. M. et al. Million Veteran Program: a mega-biobank to study genetic influences on health and disease. J. Clin. Epidemiol. 70, 214–223 (2016).
de Leeuw, C. A., Mooij, J. M., Heskes, T. & Posthuma, D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput. Biol. 11, e1004219 (2015).
Volkow, N. D. et al. The conception of the ABCD study: from substance use to a broad NIH collaboration. Dev. Cogn. Neurosci. 32, 4–7 (2018).
Satterthwaite, T. D. et al. The Philadelphia Neurodevelopmental Cohort: a publicly available resource for the study of normal and abnormal brain development in youth. Neuroimage 124, 1115–1119 (2016).
Ollier, W., Sprosen, T. & Peakman, T. UK Biobank: from concept to reality. Pharmacogenomics 6, 639–646 (2005).
Diagnostic and Statistical Manual of Mental Disorders (DSM-5) 5th edn (American Psychiatric Association Publishing, 2013).
Kessler, R. C. et al. The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biol. Psychiatry 73, 904–914 (2013).
Sonneville, K. R. & Lipson, S. K. Disparities in eating disorder diagnosis and treatment according to weight status, race/ethnicity, socioeconomic background, and sex among college students. Int. J. Eat. Disord. 51, 518–526 (2018).
Taliun, D. et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature 590, 290–299 (2021).
Polimanti, R. et al. Leveraging genome-wide data to investigate differences between opioid use vs. opioid dependence in 41,176 individuals from the Psychiatric Genomics Consortium. Mol. Psychiatry 25, 1673–1687 (2020).
Bulik-Sullivan, B. et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295 (2015).
Willer, C. J., Li, Y. & Abecasis, G. R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010).
Turley, P. et al. Multi-ancestry meta-analysis yields novel genetic discoveries and ancestry-specific associations. Preprint at bioRxiv https://doi.org/10.1101/2021.04.23.441003 (2021).
Zou, Y., Carbonetto, P., Wang, G. & Stephens, M. Fine-mapping from summary data with the ‘Sum of Single Effects’ model. PLoS Genet. 18, e1010299 (2022).
Burstein, D. et al. Detecting and adjusting for hidden biases due to phenotype misclassification in genome-wide association studies. Preprint at medRxiv https://doi.org/10.1101/2023.01.17.23284670 (2023).
Genovese, C. R., Roeder, K. & Wasserman, L. False discovery control with p-value weighting. Biometrika 93, 509–524 (2006).
Karlsson Linnér, R. et al. Multivariate analysis of 1.5 million people identifies genetic associations with traits related to self-regulation and addiction. Nat. Neurosci. 24, 1367–1376 (2021).
Williams, C. et al. Guidelines for evaluating the comparability of down-sampled GWAS summary statistics. Preprint at bioRxiv https://doi.org/10.1101/2023.03.21.533641 (2023).
Elsworth, B. et al. The MRC IEU OpenGWAS data infrastructure. Preprint at bioRxiv https://doi.org/10.1101/2020.08.10.244293 (2020).
Sudlow, C. et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015).
Zhu, Z. et al. Causal associations between risk factors and common diseases inferred from GWAS summary data. Nat. Commun. 9, 224 (2018).
Bell, S. et al. A genome-wide meta-analysis yields 46 new loci associating with biomarkers of iron homeostasis. Commun. Biol. 4, 156 (2021).
Tanimura, N. et al. GATA/heme multi-omics reveals a trace metal-dependent cellular differentiation mechanism. Dev. Cell 46, 581–594.e4 (2018).
Domcke, S. A human cell atlas of fetal chromatin accessibility. Science 370, eaba7612 (2020).
Corces, M. R. et al. Single-cell epigenomic analyses implicate candidate causal variants at inherited risk loci for Alzheimer’s and Parkinson’s diseases. Nat. Genet. 52, 1158–1168 (2020).
An, S. J., Kim, T. J. & Yoon, B.-W. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J. Stroke 19, 3–10 (2017).
Stunkard, A. J. & Allison, K. C. Binge eating disorder: disorder or marker? Int. J. Eat. Disord. 34 (Suppl.), S107–S116 (2003).
Hinckley, J. D. et al. Quantitative trait locus linkage analysis in a large Amish pedigree identifies novel candidate loci for erythrocyte traits. Mol. Genet. Genom. Med. 1, 131–141 (2013).
Galmozzi, A. et al. PGRMC2 is an intracellular haem chaperone critical for adipocyte function. Nature 576, 138–142 (2019).
Borgna-Pignatti, C. & Zanella, S. Pica as a manifestation of iron deficiency. Expert Rev. Hematol. 9, 1075–1080 (2016).
Ersche, K. D. et al. Disrupted iron regulation in the brain and periphery in cocaine addiction. Transl. Psychiatry 7, e1040 (2017).
Barnea, R. et al. Trait and state binge eating predispose towards cocaine craving. Addict. Biol. 22, 163–171 (2017).
Succurro, E. et al. Obese patients with a binge eating disorder have an unfavorable metabolic and inflammatory profile. Medicine 94, e2098 (2015).
Al-Massadi, O. et al. Multifaceted actions of melanin-concentrating hormone on mammalian energy homeostasis. Nat. Rev. Endocrinol. 17, 745–755 (2021).
Noble, E. E. et al. Hypothalamus–hippocampus circuitry regulates impulsivity via melanin-concentrating hormone. Nat. Commun. 10, 4923 (2019).
Harrington, K. M. et al. Gender differences in demographic and health characteristics of the Million Veteran Program cohort. Women’s Health Issues 29 (Suppl. 1), S56–S66 (2019).
Gelernter, J. et al. Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in >165,000 US veterans. Nat. Neurosci. 22, 1394–1401 (2019).
Fang, H. et al. Harmonizing genetic ancestry and self-identified race/ethnicity in genome-wide association studies. Am. J. Hum. Genet. 105, 763–772 (2019).
1000 Genomes Project Consortium An integrated map of genetic variation from 1,092 human genomes. Nature 491, 56–65 (2012).
Karcher, N. R. & Barch, D. M. The ABCD study: understanding the development of risk for mental and physical health outcomes. Neuropsychopharmacology 46, 131–142 (2021).
Wu, P. et al. Mapping ICD-10 and ICD-10-CM codes to phecodes: workflow development and initial evaluation. JMIR Med. Inform. 7, e14325 (2019).
Loh, P.-R. et al. Reference-based phasing using the Haplotype Reference Consortium panel. Nat. Genet. 48, 1443–1448 (2016).
1000 Genomes Project Consortium A global reference for human genetic variation. Nature 526, 68–74 (2015).
Manichaikul, A. et al. Robust relationship inference in genome-wide association studies. Bioinformatics 26, 2867–2873 (2010).
Price, A. L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Patterson, N., Price, A. L. & Reich, D. Population structure and eigenanalysis. PLoS Genet. 2, e190 (2006).
Bigdeli, T. B. et al. A simple yet accurate correction for winner’s curse can predict signals discovered in much larger genome scans. Bioinformatics 32, 2598–2603 (2016).
Watanabe, K., Taskesen, E., van Bochoven, A. & Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 8, 1826 (2017).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241 (2015).
Wang, G., Sarkar, A., Carbonetto, P. & Stephens, M. A simple new approach to variable selection in regression, with application to genetic fine mapping. J. R. Stat. Soc. B 82, 1273–1300 (2020).
Denny, J. C. et al. Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nat. Biotechnol. 31, 1102–1110 (2013).
Ge, T., Chen, C.-Y., Ni, Y., Feng, Y.-C. A. & Smoller, J. W. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat. Commun. 10, 1776 (2019).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Yang, J., Lee, S. H., Goddard, M. E. & Visscher, P. M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88, 76–82 (2011).
Churchhouse, C. & Neale, B. Rapid GWAS of Thousands of Phenotypes for 337,000 Samples in the UK Biobank http://www.nealelab.is/blog/2017/7/19/rapid-gwas-of-thousands-of-phenotypes-for-337000-samples-in-the-uk-biobank (Biobank, 2017).
Finucane, H. K. et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat. Genet. 47, 1228–1235 (2015).
Schilder, B. M., Humphrey, J. & Raj, T. echolocatoR: an automated end-to-end statistical and functional genomic fine-mapping pipeline. Bioinformatics 38, 536–539 (2021).
Acknowledgements
This research is based on data from the MVP, Office of Research and Development, Veterans Health Administration, and was supported by award no. MVP006. This publication does not represent the views of the Department of Veteran Affairs or the United States Government. This study was also supported by the National Institutes of Health (NIH), Bethesda, Maryland, USA, under award numbers T32MH087004 (K.T.), T32MH096679 (T.C.G.), T32MH122394 (A.M.), K08MH122911 (G.V.), R01MH125246, R01AG067025, U01MH116442 and R01MH109677 (P.R.), and by the Veterans Affairs Merit grants BX002395 and BX004189 (P.R.). This study was also funded in part by the Brain & Behavior Research Foundation through the 2020 NARSAD Young Investigator Grant no. 29350 (G.V.). We thank S. W. Choi and P. F. O’Reilly for their guidance and expertise in using data from the UKBB. We thank the participants in the UKBB and the scientists involved in the construction of this resource. This research was conducted using the UKBB resource under application 18177 (P. F. O’Reilly). This work was supported in part by the computational resources and staff expertise provided by Scientific Computing at the Icahn School of Medicine at Mount Sinai, New York, New York, USA. Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the National Institute of Mental Health Data Archive (NDA), Bethesda, Maryland, USA. This is a multi-site, longitudinal study designed to recruit more than 10,000 children aged 9–10 years and follow them over 10 years into early adulthood. The ABCD Study is supported by the NIH and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, U24DA041147, U01DA041093 and U01DA041025. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/scientists/workgroups. ABCD consortium investigators designed and implemented the study and/or provided data but did not participate in the analysis or writing of this report. The manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from https://doi.org/10.15154/1527728. DOIs can be found at https://nda.nih.gov/study.html?id=1661. Support for data collection for the PNC, acquired through dbGaP (accession no. phs000607.v3.p2), was provided by grant RC2MH089983 awarded to R. Gur and RC2MH089924 awarded to H. Hakonarson. Participants were recruited and genotyped through the Center for Applied Genomics (CAG) at the Children’s Hospital in Philadelphia (CHOP), Pennsylvania, USA. Phenotypic data collection occurred at the CAG and CHOP and at the Brain Behavior Laboratory, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Author information
Authors and Affiliations
Contributions
D.B., K.T., J.B., S.V., P.D., B.Z. and D.M. performed the analysis. D.B., K.T., J.B. and S.V. performed sample and/or data provision and processing. D.B. and T.C.G. wrote the manuscript. D.B., T.C.G., K.T., J.B., S.V., A.M., G.H., R.S., T.H., G.V. and P.R. performed core revision of the manuscript. T.C.G., G.H., R.S., T.H., G.V. and P.R. provided study direction. G.H., R.S., T.H., G.V. and P.R. supervised the study. All authors contributed to critical revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
T.H. is a scientific advisory board member of Noom. T.H. and R.S. receive funding from and have equity in Noom (a non-publicly traded company). R.S. receives royalties from Wolters Kluwer Health. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Eske Derks, Adam Locke and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Note and Supplementary Figs. 1–15
Supplementary Table 1
Supplementary Tables 1–14
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Burstein, D., Griffen, T.C., Therrien, K. et al. Genome-wide analysis of a model-derived binge eating disorder phenotype identifies risk loci and implicates iron metabolism. Nat Genet 55, 1462–1470 (2023). https://doi.org/10.1038/s41588-023-01464-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-023-01464-1
This article is cited by
-
Valid inference for machine learning-assisted genome-wide association studies
Nature Genetics (2024)
-
Expanding drug targets for 112 chronic diseases using a machine learning-assisted genetic priority score
Nature Communications (2024)
-
Machine learning drives genetic discovery for binge eating disorder
Nature Genetics (2023)