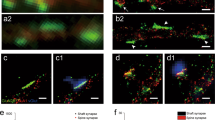
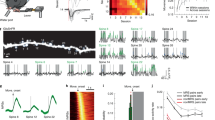
Abstract
Newly generated excitatory synapses in the mammalian cortex lack sufficient AMPA-type glutamate receptors to mediate neurotransmission, resulting in functionally silent synapses that require activity-dependent plasticity to mature. Silent synapses are abundant in early development, during which they mediate circuit formation and refinement, but they are thought to be scarce in adulthood1. However, adults retain a capacity for neural plasticity and flexible learning that suggests that the formation of new connections is still prevalent. Here we used super-resolution protein imaging to visualize synaptic proteins at 2,234 synapses from layer 5 pyramidal neurons in the primary visual cortex of adult mice. Unexpectedly, about 25% of these synapses lack AMPA receptors. These putative silent synapses were located at the tips of thin dendritic protrusions, known as filopodia, which were more abundant by an order of magnitude than previously believed (comprising about 30% of all dendritic protrusions). Physiological experiments revealed that filopodia do indeed lack AMPA-receptor-mediated transmission, but they exhibit NMDA-receptor-mediated synaptic transmission. We further showed that functionally silent synapses on filopodia can be unsilenced through Hebbian plasticity, recruiting new active connections into a neuron’s input matrix. These results challenge the model that functional connectivity is largely fixed in the adult cortex and demonstrate a new mechanism for flexible control of synaptic wiring that expands the learning capabilities of the mature brain.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data generated and analysed in the current study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
Code for image analysis can be accessed at https://github.com/harnett/FilopodiaStructuralSubstrateSilentSynapses.
References
Hanse, E., Seth, H. & Riebe, I. AMPA-silent synapses in brain development and pathology. Nat. Rev. Neurosci. 14, 839–850 (2013).
Katz, L. C. & Shatz, L. C. Synaptic activity and the construction of cortical circuits. Science 274, 1133–1138 (1996).
Magee, J. C. & Grienberger, C. Synaptic plasticity forms and functions. Annu. Rev. Neurosci. 43, 95–117 (2020).
Trachtenberg, J. T. et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420, 788–794 (2002).
Knott, G. W., Holtmaat, A., Wilbrecht, L., Welker, E. & Svoboda, K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat. Neurosci. 9, 1117–1124 (2006).
Grutzendler, J., Kasthuri, N. & Gan, W. B. Long-term dendritic spine stability in the adult cortex. Nature 420, 812–816 (2002).
Holtmaat, A. J. G. D. et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron 45, 279–291 (2005).
Fusi, S. & Abbott, L. F. Limits on the memory storage capacity of bounded synapses. Nat. Neurosci. 10, 485–493 (2007).
Poirazi, P. & Mel, B. W. Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron 29, 779–796 (2001).
Durand, G. M., Kovalchuk, Y. & Konnerth, A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature 381, 71–75 (1996).
Huang, X. et al. Progressive maturation of silent synapses governs the duration of a critical period. Proc. Natl Acad. Sci. USA 112, E3131–E3140 (2015).
Busetto, G., Higley, M. J. & Sabatini, B. L. Developmental presence and disappearance of postsynaptically silent synapses on dendritic spines of rat layer 2/3 pyramidal neurons. J. Physiol. 586, 1519–1527 (2008).
Isaac, J. T. R., Crair, M. C., Nicoll, R. A. & Malenka, R. C. Silent synapses during development of thalamocortical inputs. Neuron 18, 269–280 (1997).
Anastasiades, P. G. & Butt, S. J. B. A role for silent synapses in the development of the pathway from layer 2/3 to 5 pyramidal cells in the neocortex. J. Neurosci. 32, 13085–13099 (2012).
Park, J. et al. Epitope-preserving magnified analysis of proteome (eMAP). Sci. Adv. 7, eabf6589–eabf6589 (2021).
Fiala, J. C., Feinberg, M., Popov, V. & Harris, K. M. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J. Neurosci. 18, 8900–8911 (1998).
Zuo, Y., Lin, A., Chang, P. & Gan, W. B. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron 46, 181–189 (2005).
Dunaevsky, A., Tashiro, A., Majewska, A., Mason, C. & Yuste, R. Developmental regulation of spine motility in the mammalian central nervous system. Proc. Natl Acad. Sci. USA 96, 13438–13443 (1999).
Berry, K. P. & Nedivi, E. Spine dynamics: are they all the same? Neuron 96, 43–55 (2017).
Gundelfinger, E. D., Reissner, C. & Garner, C. C. Role of Bassoon and Piccolo in assembly and molecular organization of the active zone. Front. Synaptic Neurosci. 7, 19 (2016).
Nusser, Z. et al. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron 21, 545–559 (1998).
Kharazia, V. N. & Weinberg, R. J. Immunogold localization of AMPA and NMDA receptors in somatic sensory cortex of albino rat. J. Comp. Neurol. 412, 292–302 (1999).
Holler, S., Köstinger, G., Martin, K. A. C., Schuhknecht, G. F. P. & Stratford, K. J. Structure and function of a neocortical synapse. Nature 591, 111–116 (2021).
Liao, D., Scannevin, R. H. & Huganir, R. Activation of silent synapses by rapid activity-dependent synaptic recruitment of AMPA receptors. J. Neurosci. 21, 6008–6017 (2001).
Isaac, J. T. R., Nicoll, R. A. & Malenka, R. C. Evidence for silent synapses: implications for the expression of LTP. Neuron 15, 427–434 (1995).
Mainen, Z. F., Malinow, R. & Svoboda, K. Synaptic calcium transients in single spines indicate that NMDA receptors are not saturated. Nature 399, 151–155 (1999).
Oertner, T. G., Sabatini, B. L., Nimchinsky, E. A. & Svoboda, K. Facilitation at single synapses probed with optical quantal analysis. Nat. Neurosci. 5, 657–664 (2002).
Kerchner, G. A. & Nicoll, R. A. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat. Rev. Neurosci. 9, 813–825 (2008).
Lisman, J. E., Raghavachari, S. & Tsien, R. W. The sequence of events that underlie quantal transmission at central glutamatergic synapses. Nat. Rev. Neurosci. 8, 597–609 (2007).
Liao, D., Hessler, N. A. & Malinow, R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature 375, 400–404 (1995).
Zhu, J. J., Esteban, J. A., Hayashi, Y. & Malinow, R. Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat. Neurosci. 3, 1098–1106 (2000).
Tazerart, S., Mitchell, D. E., Miranda-Rottmann, S. & Araya, R. A spike-timing-dependent plasticity rule for dendritic spines. Nat. Commun. 11, 4276 (2020).
Wassie, A. T., Zhao, Y. & Boyden, E. S. Expansion microscopy: principles and uses in biological research. Nat. Methods 16, 33–41 (2019).
Ofer, N., Berger, D. R., Kasthuri, N., Lichtman, J. W. & Yuste, R. Ultrastructural analysis of dendritic spine necks reveals a continuum of spine morphologies. Dev. Neurobiol. 81, 746–757 (2021).
Kullmann, D. M. Amplitude fluctuations of dual-component EPSCs in hippocampal pyramidal cells: implications for long-term potentiation. Neuron 12, 1111–1120 (1994).
Wright, W. J. et al. Silent synapses dictate cocaine memory destabilization and reconsolidation. Nat. Neurosci. 23, 32–46 (2020).
Takumi, Y., Ramírez-León, V., Laake, P., Rinvik, E. & Ottersen, O. P. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat. Neurosci. 2, 618–624 (1999).
Benna, M. K. & Fusi, S. Computational principles of synaptic memory consolidation. Nat. Neurosci. 19, 1697–1706 (2016).
Fusi, S., Drew, P. J. & Abbott, L. F. Cascade models of synaptically stored memories. Neuron 45, 599–611 (2005).
Aitchison, L. et al. Synaptic plasticity as Bayesian inference. Nat. Neurosci. 24, 565–571 (2021).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
Yang, G., Chang, P. C., Bekker, A., Blanck, T. J. J. & Gan, W. B. Transient effects of anesthetics on dendritic spines and filopodia in the living mouse cortex. Anesthesiology 115, 718–726 (2011).
Ji, N., Magee, J. C. & Betzig, E. High-speed, low-photodamage nonlinear imaging using passive pulse splitters. Nat. Methods 5, 197–202 (2008).
Harnett, M. T., Xu, N. L., Magee, J. C. & Williams, S. R. Potassium channels control the interaction between active dendritic integration compartments in layer 5 cortical pyramidal neurons. Neuron 79, 516–529 (2013).
Matsuzaki, M., Honkura, N., Ellis-Davies, G. C. R. & Kasai, H. Structural basis of long-term potentiation in single dendritic spines. Nature 255, 243–244 (2004).
Weber, J. P. et al. Location-dependent synaptic plasticity rules by dendritic spine cooperativity. Nat. Commun. 7, 11380 (2016).
Acknowledgements
We thank D. H. Yun for technical assistance with eMAP, K. Tsimring and A. Krol for technical assistance with perfusions, and C. Yaeger, M. Tadross, E. Nedivi and M. Bear for constructive criticism of the manuscript. We thank H. Umemori for the donation of Thy1-GFP-M+ mouse pups. Financial support was provided by the Boehringer Ingelheim Fonds (D.V.), National Institutes of Health RO1NS106031 (M.T.H.), the James W. and Patricia T. Poitras Fund at MIT (M.T.H.), a Klingenstein-Simons Fellowship (M.T.H.), a Vallee Foundation Scholarship (M.T.H.) and a McKnight Scholarship (M.T.H.).
Author information
Authors and Affiliations
Contributions
D.V. performed all experiments, analysed all data and prepared the figures. K.C. provided eMAP resources. M.T.H supervised all aspects of the project and wrote the manuscript with D.V.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Bernardo Sabatini and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Morphological measurements of dendritic protrusions.
a, Illustration of a dendritic protrusion and corresponding measurements: head diameter. (dhead), neck diameter (dneck), length (l). b–d, Population histograms of morphological characteristics across all dendritic protrusions (n = 2234). e, Population histogram of the relationship between dhead and dneck. Shaded area indicates a ratio below 1.3, the first criterion used to classify filopodia versus spines. f, Population histogram of dhead/l for protrusions with dhead/dneck below 1.3 (shaded area in e). Protrusions with dhead/l above 3 were classified as filopodia, those below 3 were likely short stubby spines and were not analyzed further (shaded area= (dhead /dneck < 1.3) ∩ (l/ dhead>3)). g, Same as e but for each of the 4 mice. h, Fraction of dendritic protrusions classified as filopodia per mouse (n = 527, 944, 435, 328 dendritic protrusions and 30, 47, 25, 21 dendritic branches for mouse 1, 2, 3, 4 respectively). Box plot represents median and IQR with whiskers extending to the most extreme points not considered outliers. ns P = 0.093, Kruskal-Wallis test.
Extended Data Fig. 2 Filopodia in L2/3 pyramidal neurons exhibit AMPAR immune-negative and NMDAR immune-positive synapses.
a, Example confocal image of a V1 L2/3 neuron (green arrowhead) expressing GFP after viral transfection in V1 in an originally 45 μm thick slice. Scale bar: 100 μm expanded/ 59 μm original. Image was taken after reshrinking the tissue from 4x expansion to 1.7x expansion. b, Box plot (left) and kernel density estimate (right) of signal intensity in Bassoon (blue), NMDAR (yellow), and AMPAR (red) channels for L2/3 pyramidal neuron spines (n = 275). Box plot represents median and IQR with whiskers extending to the most extreme points not considered outliers. Signal in each channel is shown for all dendritic protrusions, each represented by one dot. c, As in b, but for filopodia (n = 134).
Extended Data Fig. 3 Anti-Bassoon signal intensity threshold for presence of presynaptic partner.
a, Cumulative density function of Bassoon signal intensity in spines (red) and filopodia (yellow). Vertical line at the choosen threshold (anti-Bassoon signal = 0). b, Magnified plot of a around 0. c-d, Example filopodia with anti-Bassoon signal intensities below above the threshold. Scale bar: 5 μm expanded/1.25 μm original.
Extended Data Fig. 4 Representatitive examples of eMAP at dendritic protrusions.
Example four channel images of dendritic protrusions with different dhead/dneck values (increasing from left to right). From top to bottom: cell-filling GFP stained with Alexa Fluor 488 (green) at lower magnification to show full protrusion shape, presynaptic protein Bassoon stained Alexa Fluor 405 (blue), NMDAR subunit NR1(GluN1) stained with Alexa Fluor 555 (yellow), and AMPAR subunit GluR1(GluA1) stained with Alexa Fluor 647 (red), all at higher magnifiaction to show synaptic localization. Scale bar: 2 μm expanded/0.5 μm original.
Extended Data Fig. 5 Anti-GluA1 signal increases with spine size.
a, Anti-GluA1 signal intensity as a function of head diameter for spines (red) and filopodia (yellow). b, Correlation between head diameter and anti-GluA1 signal intensity for spines. The data are fitted with a line of slope 421+/−22 using linear regression. c, Correlation between diameter of filopodium head and anti-GluaA1 signal intensity. The data are fitted with a line of slope 95+/−39 using linear regression. Correlation coefficients (r) and p-values were obtained from a two-tailed, non-parametric Spearman correlation.
Extended Data Fig. 6 Spatial resolution of two-photon MNI-glutamate uncaging at adult mouse cortical protrusions.
a, Left: Two-photon z-stack of a V1 L5 pyramidal neuron filled with Alexa-488 via somatic patch pipette. Basal branch segment of interest indicated by yellow box. Right: Magnified view of basal branch of interest. b, (top) Voltage response for the spine at lateral uncaging locations shown in a. (bottom) plot of lateral uncaging resolution. Continuous line is the Gaussian fit of the amplitudes of two-photon glutamate uncaging along lateral steps (circles). c, (top) Voltage response for the spine at axial locations shown in a. Each voltage trace is an average of the voltage traces evoked at a specific axial step above and below of the spine. (bottom) plot of axial uncaging resolution (see b). d, Magnified view of a filopodium of a basal branch of a L5 pyramidal neuron. All uncaging experiments shown in e and f were performed in Mg2+ free ASCF with AMPA blocked (DNQX, 20 μM). e, As in b, for the filopodium shown in d. f, As in c, for the filopodium shown in d.
Extended Data Fig. 7 Responses to focal extracellular synaptic stimulation for the filopodium shown in Fig. 3e.
a, Superimposed traces of somatic voltage recordings (left) and corresponding changes in local Ca2+ (measured via Fluo-4 fluorescence; ΔF/F) at the parent dendritic branch (middle) and at the tip of the filopodium (right) in response to focal extracellular synaptic stimulation in Mg2+-free aCSF with AMPA blocked (via DNQX, 20 μΜ). All synaptic stimulation successes and failures for the filopodium in Fig. 3e are shown. Synaptic stimulation driven backpropagating action potential (bAP) shown in red. b, Same as in a with traces spaced apart. Grey dashed line indicates the onset of synaptic stimulation.
Extended Data Fig. 8 Length of protrusions before and after induction protocols.
Length of protrusions before (grey) and after (red) induction in filopodia and spines. Three different induction protocols were tested in filopodia: i- Pairing protocol (n = 15 filopodia from 13 slices and 10 mice); ii- Somatic action potentials without any caged glutamate present (Post alone; n = 7 filopodia from 7 slices and 6 mice); iii- Glutamate uncaging without somatic action potential (Pre alone; n = 7 filopodia from 7 slices and 6 mice); ns P > 0.15. Two-sided Wilcoxon signed-rank test. Box plot represents median and IQR with whiskers extending to the most extreme points not considered outliers.
Extended Data Fig. 9 Spiny synapses do not exhibit changes in synaptic strength or length in response to the STDP protocol.
a, Schematic of the experiment. A control spine on a different branch than the branch of the test spine was always present. 40 and 90 repetitions of the pairing protocol were used for spines. b, Relative change of peak somatic uEPSP amplitude after pairing. P = 0.5781 (40 repetitions, n = 7 test and 7 control spines from 7 slices and 4 mice), P = 0.9375 (90 repetitions, n = 7 test and 7 control spines from 7 slices and 3 mice), two-sided Wilcoxon signed-rank test. Box plot represents median and IQR with whiskers extending to the 95% CI. c, Relative change of spine length after pairing. P = 0.4688 (40 repetitions, n = 7 test and 7 control spines from 7 slices and 4 mice), P = 0.8125 (90 repetitions, n = 7 test and 7 control spines from 7 slices and 3 mice), two-sided Wilcoxon signed-rank test. Box plot represents median and IQR with whiskers extending to the 95% CI.
Extended Data Fig.10 Super-resolution characterization of synapses in developing mouse visual cortex.
a, Example confocal image of a postnatal day (P) 13 Thy1- GFP-M+ L5 pyramidal neuron dendritic segment after 4x expansion. Scale bar: 10 μm expanded/2.5 μm original. b, Fraction of dendritic protrusions classified as filopodia in P13 L5 PNs (n = 371 dendritic protrusion, 18 dendritic branches, 3 mice). Box plot represents median and IQR with whiskers extending to the most extreme points not considered outliers. c, Fraction of total synapses in the three dendritic locations in P13 L5 PNs (n = 397 synapses, 18 dendritic branches, 3 mice). Box plot represents median and IQR with whiskers extending to the most extreme points not considered outliers. d, (left) Box plot and individual data for signal intensity in Bassoon (blue), NMDAR (yellow), and AMPAR (red) channels for spines (n = 236). (right) example four channel images of a representative spine. Box plot represents median and IQR with whiskers extending to the most extreme points not considered outliers. Scale bar: 5 μm expanded/1.25 μm original (GFP), 1 μm expanded/0.25 μm original (Bassoon). e, As in b, but for filopodia (n = 79). f, As in b, but for shaft synapses (n = 82). Example images show a shaft synapse that lacks AMPARs (top) and a shaft synapse that exhibits AMPARs (bottom). g, Comparison of dendritic protrusion types in P13 (n = 371) and adult mice (n = 2234). h, Comparison of synapse distribution in P13 (n = 397) and adult mice (n = 2188).
Supplementary information
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vardalaki, D., Chung, K. & Harnett, M.T. Filopodia are a structural substrate for silent synapses in adult neocortex. Nature 612, 323–327 (2022). https://doi.org/10.1038/s41586-022-05483-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05483-6
This article is cited by
-
A perspective on Alzheimer’s disease: exploring the potential of terminal/paradoxical lucidity and psychedelics
Molecular Neurodegeneration (2024)
-
Liprin-α proteins are master regulators of human presynapse assembly
Nature Neuroscience (2024)
-
Combined expansion and STED microscopy reveals altered fingerprints of postsynaptic nanostructure across brain regions in ASD-related SHANK3-deficiency
Molecular Psychiatry (2024)
-
Metabotropic signaling within somatostatin interneurons controls transient thalamocortical inputs during development
Nature Communications (2024)
-
Tau in cerebrospinal fluid induces neuronal hyperexcitability and alters hippocampal theta oscillations
Acta Neuropathologica Communications (2023)