Key Points
-
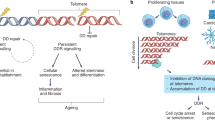
Telomeres are specialized chromatin structures at the ends of chromosomes, which consist of tandem DNA repeats (of TTAGGG) and associated proteins. Telomeres are also bound by nucleosome arrays, which contain epigenetic modifications that are characteristic of constitutive heterochromatin.
-
Telomeres protect chromosome ends from repair and degradation activities. This function is impaired by both the shortening of TTAGGG repeats to below a critical length, and the loss of telomere-binding proteins.
-
Dysfunctional telomeres trigger a DNA damage response, which results in cell-cycle arrest or apoptosis. Telomere dysfunction can also lead to end-to-end chromosome fusions, and can interfere with the repair of DNA lesions in non-telomeric regions, resulting in hypersensitivity to various genotoxic agents.
-
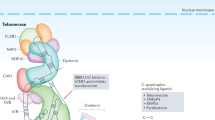
Telomerase is a cellular reverse transcriptase that synthesizes de novo telomeric repeats at chromosome ends. Most somatic tissues lack telomerase activity and show progressive telomere shortening coupled to cell division.
-
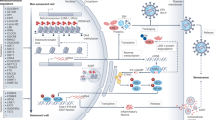
Various diseases associated with ageing, including cancer, as well as a number of premature ageing syndromes, are characterized by critically short telomeres. Telomere shortening and the absence of telomerase in normal tissues is a tumour-suppression mechanism. By contrast, tumours aberrantly upregulate telomerase, which elongates short telomeres and allows continuous growth.
-
Mice that lack telomerase activity age prematurely and are more resistant to cancer. Human premature ageing syndromes that are characterized by short telomeres are recapitulated in the mouse only when in the context of telomerase-deficiency and short telomeres.
-
The telomerase core components telomerase reverse transcriptase (TERT) and telomerase RNA component (TERC), as well as telomerase-interacting protein dyskeratosis congenita 1, dyskerin (DKC1), are mutated in the premature ageing human diseases dyskeratosis congenita and aplastic anaemia.
-
Several telomere-binding proteins are altered in human cancer and premature ageing syndromes that are characterized by chromosomal instability.
-
Telomerase and telomere-binding proteins are new potential targets for anti-cancer and anti-ageing therapies.
Abstract
Telomere length and telomerase activity are important factors in the pathobiology of human disease. Age-related diseases and premature ageing syndromes are characterized by short telomeres, which can compromise cell viability, whereas tumour cells can prevent telomere loss by aberrantly upregulating telomerase. Altered functioning of both telomerase and telomere-interacting proteins is present in some human premature ageing syndromes and in cancer, and recent findings indicate that alterations that affect telomeres at the level of chromatin structure might also have a role in human disease. These findings have inspired a number of potential therapeutic strategies that are based on telomerase and telomeres.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
De Lange, T. Protection of mammalian telomeres. Oncogene 21, 532–540 (2002).
Blackburn, E. H. Switching and signaling at the telomere. Cell 106, 661–673 (2001).
Collins, K. & Mitchell, J. R. Telomerase in the human organism. Oncogene 21, 564–579 (2002).
Griffith, J. D. et al. Mammalian telomeres end in a large duplex loop. Cell 97, 503–514 (1999).
Nikitina, T. & Woodcock, C. L. Chromatin loops at the ends of chromosomes. J. Cell Biol. 166, 161–165 (2004).
De Lange, T. T-loops and the origin of telomeres. Nature Rev. Mol. Cell Biol. 5, 323–329 (2004). A must-read review on the origins of telomeres and the formation of telomeric loops (T loops). A clear connection between T-loop formation and homologous recombination mechanisms is proposed.
Tommerup, H., Dousmanis, A. & de Lange, T. Unusual chromatin in human telomeres. Mol. Cell. Biol. 14, 5777–5785 (1994).
Garcia Cao, M. et al. Epigenetic regulation of telomere length in mammalian cells by the Suv39h1 and Suv39h2 histone methyltransferases. Nature Genet. 36, 94–99 (2004). This paper showed that mammalian telomeres contain the main marks of constitutive heterochromatin and that epigenetic modifications represent a higher-order telomere-length control mechanism in mammals.
Gonzalo, S. et al. Role of the RB1 family in stabilizing histone methylation at constitutive heterochromatin. Nature Cell Biol. 7, 420–428 (2005).
Peters, A. H. et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107, 323–337 (2001).
Schotta, G. et al. A silencing pathway to induce H3-K9 and H4-K20 trimethylation at constitutive heterochromatin. Genes Dev. 8, 1251–1262 (2004).
Garcia-Cao, M., Gonzalo, S., Dean, D. & Blasco, M. A. A role for the Rb family of proteins in controlling telomere length. Nature Genet. 32, 415–419 (2002).
Perrod, S. & Gasser, S. M. Long-range silencing and position effects at telomeres and centromeres: parallels and differences. Cell. Mol. Life Sci. 60, 2303–2318 (2003).
Baur, J. A., Zou, Y., Shay, J. W. & Wright, W. E. Telomere position effect in human cells. Science 292, 2075–2077 (2001). The authors provide conclusive evidence that telomere position effect (the silencing of genes near the telomeres) operates in mammalian cells.
Koering, C. E. et al. Human telomeric position effect is determined by chromosomal context and telomeric chromatin integrity. EMBO Rep. 3, 1055–1061 (2002).
Shiels, P. G. et al. Analysis of telomere lengths in cloned sheep. Nature 399, 316–317 (1999).
Lanza, R. P. et al. Extension of cell life-span and telomere length in animals cloned from senescent somatic cells. Science 288, 665–669 (2000).
Ahmad, K. & Henikoff, S. Epigenetic consequences of nucleosome dynamics. Cell 111, 281–284 (2002).
Smogorzewska, A. & de Lange, T. Regulation of telomerase by telomeric proteins. Annu. Rev. Biochem. 73, 177–208 (2004).
Karlseder, J. et al. Targeted deletion reveals an essential function for the telomere length regulator Trf1. Mol. Cell. Biol. 23, 6533–6541 (2003).
Dynek, J. N. & Smith, S. Resolution of sister telomere association is required for progression through mitosis. Science 304, 97–100 (2004). This paper provides evidence for the role of telomere-binding proteins in sister chromatid cohesion, therefore linking telomere function with cell division.
Chiang, Y. J., Kim, S. H., Tessarollo, L., Campisi, J. & Hodes, R. J. Telomere-associated protein TIN2 is essential for early embryonic development through a telomerase-independent pathway. Mol. Cell. Biol. 15, 6631–6634 (2004).
Kaminker, P. et al. Higher-order nuclear organization in growth arrest of human mammary epithelial cells: a novel role for telomere-associated protein TIN2. J. Cell Sci. 118, 1321–1330 (2005).
Oh, B. K., Kim, Y. J., Park, C. & Park, Y. N. Up-regulation of telomere-binding proteins, TRF1, TRF2, and TIN2 is related to telomere shortening during human multistep hepatocarcinogenesis. Am. J. Pathol. 166, 73–80 (2005). Telomere repeat binding proteins are shown to be vastly upregulated in human cancer, which indicates that these proteins could have a role in promoting tumorigenesis.
Gelmini, S. et al. Tankyrase, a positive regulator of telomere elongation, is over expressed in human breast cancer. Cancer Lett. 216, 81–87 (2004).
Kondo, T. et al. Expression of POT1 is associated with tumor stage and telomere length in gastric carcinoma. Cancer Res. 64, 523–529 (2004).
Miyachi, K. et al. Correlation between telomerase activity and telomeric-repeat binding factors in gastric cancer. J. Exp. Clin. Cancer Res. 21, 269–275 (2002).
Matsutani, N. et al. Expression of telomeric repeat binding factor 1 and 2 and TRF1-interacting nuclear protein 2 in human gastric carcinomas. Int. J. Oncol. 19, 507–512 (2001).
Zhu, X. D. et al. ERCC1/XPF removes the 3′ overhang from uncapped telomeres and represses formation of telomeric DNA-containing double minute chromosomes. Mol. Cell 12, 1489–1498 (2003).
Dantzer, F. et al. Functional interaction between poly(ADP-ribose) polymerase 2 (PARP-2) and TRF2: PARP activity negatively regulates TRF2. Mol. Cell. Biol. 24, 1595–1607 (2004).
Opresko, P. L. et al. The Werner syndrome helicase and exonuclease cooperate to resolve telomeric D loops in a manner regulated by TRF1 and TRF2. Mol. Cell 14, 763–774 (2004).
Samper, E et al. Mammalian Ku86 protein prevents telomeric fusions independently of the length of TTAGGG repeats and the G-strand overhang. EMBO Rep. 1, 244–252 (2000).
Goytisolo, F. A. et al. The absence of the DNA-dependent protein kinase catalytic subunit in mice results in anaphase bridges and in increased telomeric fusions with normal telomere length and G-strand overhang. Mol. Cell. Biol. 21, 3642–3651 (2001).
Tarsounas, M. et al. Telomere maintenance requires the RAD51D recombination/repair protein. Cell 117, 337–347 (2004).
Jaco, I. et al. Role of mammalian Rad54 in telomere length maintenance. Mol. Cell. Biol. 23, 5572–5580 (2003).
Wang, R. C., Smogorzewska, A. & de Lange, T. Homologous recombination generates T-loop-sized deletions at human telomeres. Cell 119, 355–368 (2004). References 34–36 show a clear involvement of homologous recombination activities in telomere integrity and telomere length regulation.
Bailey, S. M. et al. Strand-specific postreplicative processing of mammalian telomeres. Science 293, 2462–2465 (2001).
Crabbe, L., Verdun, R. E., Haggblom, C. I. & Karlseder, J. Defective telomere lagging strand synthesis in cells lacking WRN helicase activity. Science 306, 1951–1953 (2004).
Espejel, S. et al. Shorter telomeres, accelerated ageing and increased lymphoma in DNA-PKcs-deficient mice. EMBO Rep. 5, 503–509 (2004).(2002a).
Espejel, S. et al. Mammalian Ku86 mediates chromosomal fusions and apoptosis caused by critically short telomeres. EMBO J. 21, 2207–2219 (2002a).
Espejel, S. et al. Functional interaction between DNA-PKcs and telomerase in telomere length maintenance. EMBO J. 21, 6275–6287 (2002b).
Bechter, O. E. et al. Telomeric recombination in mismatch repair deficient human colon cancer cells after telomerase inhibition. Cancer Res. 64, 3444–3451 (2004).
Bradshaw, P. S., Stavropoulos, D. J. & Meyn, M. S. Human telomeric protein TRF2 associates with genomic double-strand breaks as an early response to DNA damage. Nature Genet. 37, 193–197 (2005). This paper shows that TRF2 can localize to non-telomeric DNA damage lesions produced by a laser track, which indicates a role for TRF2 in DNA damage repair.
Karlseder, J. et al. The telomeric protein TRF2 binds the ATM kinase and can inhibit the ATM-dependent DNA damage response. PLoS Biol. 2, e240 (2004).
Takai, H., Smogorzewska, A. & de Lange, T. DNA damage foci at dysfunctional telomeres. Curr. Biol. 13, 1549–1556 (2003).
d'Adda di Fagagna, F. et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 (2003).
Lustig, A. J. Clues to catastrophic telomere loss in mammals from yeast telomere rapid deletion. Nature Rev. Genet. 4, 916–923 (2003).
Goytisolo, F. A. et al. Short telomeres result in organismal hypersensitivity to ionizing radiation in mammals. J. Exp. Med. 192, 1625–1636 (2000).
Ranganathan, V. et al. Rescue of a telomere length defect of Nijmegen breakage syndrome cells requires NBS and telomerase catalytic subunit. Curr. Biol. 11, 962–966 (2001).
Taylor, A. M., Groom, A. & Byrd, P. J. Ataxia-telangiectasia-like disorder (ATLD) — its clinical presentation and molecular basis. DNA repair 3, 1219–1225 (2004).
Wyllie, F. S. et al. Telomerase prevents the accelerated cell ageing of Werner syndrome fibroblasts. Nature Genet. 24, 16–17 (2000).
de Boer, J. & Hoeijmakers, J. H. Nucleotide excision repair and human syndromes. Carcinogenesis 21, 453–460 (2000).
Hande, M. P. et al. Extra-chromosomal telomeric DNA in cells from Atm−/− mice and patients with ataxia-telangiectasia. Hum. Mol. Genet. 10, 519–528 (2001).
Holgersson, A., Nilsson, A., Lewensohn, R. & Kanter, L. Expression of DNA-PKcs and Ku86, but not Ku70, differs between lymphoid malignancies. Exp. Mol. Pathol. 77, 1–6 (2004).
Gonzalez, R. et al. Loss of heterozygosity at RAD51, RAD52, RAD54 and BRCA1 and BRCA2 loci in breast cancer: pathological correlations. Br. J. Cancer 81, 503–509 (1999).
Harley, C. B., Futcher, A. B. & Greider, C. W. Telomeres shorten during ageing of human fibroblasts. Nature 345, 458–460 (1990).
Shay, J. W. & Wright, W. E. Telomerase: a target for cancer therapeutics. Cancer Cell 2, 257–265 (2002).
Lin, S. Y. & Elledge, S. J. Multiple tumor suppressor pathways negatively regulate telomerase. Cell 113, 881–889 (2003).
Blasco, M. A. Telomerase beyond telomeres. Nature Rev. Cancer 2, 627–632 (2002).
Henson, J. D., Neumann, A. A., Yeager, T. R. & Reddel, R. R. Alternative lengthening of telomeres in mammalian cells. Oncogene 21, 598–610 (2002).
Lundblad, V. Telomere maintenance without telomerase. Oncogene 21, 522–531 (2002).
Dunham, M. A., Neumann, A. A., Fasching, C. L. & Reddel, R. R. Telomere maintenance by recombination in human cells. Nature Genet. 26, 447–450 (2000).
Blasco, M. A., Funk, W. D., Villeponteau, B. & Greider, C. W. Functional characterization and developmental regulation of mouse telomerase RNA. Science 269, 1267–1270 (1995).
Blasco, M. A. et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91, 25–34 (1997).
Lee, H. W. et al. Essential role of mouse telomerase in highly proliferative organs. Nature 392, 569–574 (1998).
Blasco, M. A. Mice with bad ends: mouse models for the study of telomeres and telomerase in cancer and aging. EMBO J. 24, 1095–1103 (2005).
Herrera, E. et al. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO J. 18, 2950–2960 (1999a).
Herrera, E., Samper, E. & Blasco, M. A. Telomere shortening in mTR−/− embryos is associated with failure to close the neural tube. EMBO J. 18, 1172–1181 (1999b).
Herrera, E., Martinez, A. C. & Blasco, M. A. Impaired germinal center reaction in mice with short telomeres. EMBO J. 19, 472–481 (2000).
Franco, S., Segura, I., Riese, H. & Blasco, M. A. Decreased B16F10 melanoma growth and impaired vascularization in telomerase-deficient mice with critically short telomeres. Cancer Res. 62, 552–559 (2002).
Ferron, S. et al. Telomere shortening and chromosomal instability abrogates proliferation of adult but not embryonic neural stem cells. Development 131, 4059–4070 (2004).
Leri, A. et al. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J. 22, 131–139 (2003).
Samper, E. et al. Long-term repopulating ability of telomerase-deficient murine hematopoietic stem cells. Blood 99, 2767–2775 (2002).
Gonzalez-Suarez, E., Samper, E., Flores, J. M. & Blasco, M. A. Telomerase-deficient mice with short telomeres are resistant to skin tumorigenesis. Nature Genet. 26, 114–117 (2000). This manuscript showed for the first time that short telomeres in the absence of telomerase provide a potent tumour-suppression mechanism in response to carcinogenic treatments.
Poch, E. et al. Short telomeres protect from diet-induced atherosclerosis in apolipoprotein E-null mice. FASEB J. 18, 418–420 (2004).
Samper, E., Flores, J. M. & Blasco, M. A. Restoration of telomerase activity rescues chromosomal instability and premature aging in Terc −/− mice with short telomeres. EMBO Rep. 2, 1–8 (2001). This paper demonstrates that short telomeres in the absence of telomerase are the direct cause of chromosomal aberrations and premature ageing in the context of the telomerase-deficient mouse model, as both premature ageing and chromosomal instability can be prevented by telomerase reintroduction and rescue of short telomeres.
Chin, L. et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97, 527–538 (1999).
Rudolph, K. L., Millard, M., Bosenberg, M. W. & DePinho, R. A. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nature Genet. 28, 155–159 (2001).
Greenberg, R. A. et al. Short dysfunctional telomeres impair tumorigenesis in the INK4a(δ2/3) cancer-prone mouse. Cell 97, 515–525 (1999).
Gonzalez-Suarez, E. et al. Increased epidermal tumors and increased skin wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes. EMBO J. 20, 2619–2630 (2001).
González-Suárez, E., Flores, J. M. & Blasco, M. A. Cooperation between p53 mutation and high telomerase transgenic expression in spontaneous cancer development. Mol. Cell. Biol. 22, 7291–7301 (2002).
González-Suárez, E., Geserick, C., Flores, J. M. & Blasco, M. A. Antagonistic effects of telomerase on cancer and aging in K5-mTert transgenic mice. Oncogene 24, 2256–22570 (2005).
Artandi, S. E. et al. Constitutive telomerase expression promotes mammary carcinomas in aging mice. Proc. Natl Acad. Sci. USA 99, 8191–8196 (2002).
Canela, A., Martín-Caballero, J., Flores, J. M. & Blasco, M. A. Constitutive expression of Tert in thymocytes leads to increased incidence and dissemination of T-cell lymphoma in Lck-Tert mice. Mol. Cell. Biol. 24, 4275–4293 (2004).
Oh, H. et al. Telomerase reverse transcriptase promotes cardiac muscle cell proliferation, hypertrophy, and survival. Proc. Natl Acad. Sci. USA 98, 10308–10313 (2001).
Cayuela, M. L., Flores, J. M. & Blasco, M. A. The telomerase RNA component Terc is required for the tumour-promoting effects of Tert overexpression. EMBO Rep. 6, 268–274 (2005).
Oh, H. et al. Telomere attrition and Chk2 activation in human heart failure. Proc. Natl Acad. Sci. USA 100, 5378–5383 (2003).
O'Sullivan, J. N. et al. Chromosomal instability in ulcerative colitis is related to telomere shortening. Nature Genet. 32, 280–284 (2002).
Wiemann, S. U. et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 16, 935–942 (2002).
Samani, N. J., Boultby, R., Butler, R., Thompson, J. R. & Goodall, A. H. Telomere shortening in atherosclerosis. Lancet 358, 472–473 (2001).
Cawthon, R. M., Smith, K. R., O'Brien, E., Sivatchenko, A. & Kerber, R. A. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361, 393–365 (2003). This paper shows that telomere length in aged individuals is predictive of the time to death caused by heart disease and infections.
Epel, E. S. et al. Accelerated telomere shortening in response to life stress. Proc. Natl Acad. Sci. USA 101, 17312–17315 (2004). A negative correlation between telomerase activity levels and telomere length in blood cells is described for women exposed to perceived stress.
Mitchell, J. R., Wood, E. & Collins, K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature 402, 551–555 (1999).
Vulliamy, T. et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature 413, 432–435 (2001).
Vulliamy, T. et al. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nature Genet. 36, 447–449 (2004). A correlation between disease presentation and telomere length is observed in families with dyskeratosis congenita.
Yamaguchi, H. et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N. Engl. J. Med. 352, 1413–1424 (2005).
Marrone, A. et al. Heterozygous telomerase RNA mutations found in dyskeratosis congenita and aplastic anemia reduce telomerase activity via haploinsufficiency. Blood 104, 3936–3342 (2004).
Tahara, H. et al. Abnormal telomere dynamics of B-lymphoblastoid cell strains from Werner's syndrome patients transformed by Epstein–Barr virus. Oncogene 15, 1911–1920 (1997).
Tchirkov, A. & Lansdorp, P. M. Role of oxidative stress in telomere shortening in cultured fibroblasts from normal individuals and patients with ataxia-telangiectasia. Hum. Mol. Genet. 12, 227–327 (2003).
Lebel, M & Leder, P. A deletion within the murine Werner syndrome helicase induces sensitivity to inhibitors of topoisomerase and loss of cellular proliferative capacity. Proc. Natl Acad. Sci. USA 95, 13097–13102 (1998).
Chester, N., Kuo, F., Kozak, C., O'Hara, C. D. & Leder, P. Stage-specific apoptosis, developmental delay, and embryonic lethality in mice homozygous for a targeted disruption in the murine Bloom's syndrome gene. Genes Dev. 12, 3382–3393 (1998).
Barlow, C. et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86, 159–171 (1996).
Koomen, M. et al. Reduced fertility and hypersensitivity to mitomycin C characterize Fancg/Xrcc9 null mice. Hum. Mol. Genet. 11, 273–281 (2002).
Chang, S. et al. Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nature Genet. 36, 877–882 (2004).
Wong, K. K. et al. Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature 421, 643–648 (2003).
Du, X. et al. Telomere shortening exposes functions for the mouse Werner and Bloom syndrome genes. Mol. Cell. Biol. 24, 8437–8446 (2004).
Franco, S. et al. Telomere dynamics in Fancg-deficient mouse and human cells. Blood 104, 3927–3935 (2004).
Mochizuki, Y. et al. Mouse dyskerin mutations affect accumulation of telomerase RNA and small nucleolar RNA, telomerase activity, and ribosomal RNA processing. Proc. Natl Acad. Sci. USA 101, 10756–10761 (2004).
Espejel, S. et al. Impact of telomerase ablation on organismal viability, aging, and tumorigenesis in mice lacking the DNA repair proteins PARP-1, Ku86, or DNA-PKcs. J. Cell Biol. 167, 627–638 (2004).
Shay, J. W. & Wright, W. E. Mechanism-based combination telomerase inhibition therapy. Cancer Cell 7, 1–2 (2005).
Acknowledgements
I would like to thank P. Muñoz, S. Gonzalo and I. Flores for critical reading of this manuscript. M.A.B.'s laboratory is funded by the Spanish Ministery of Culture and Science, the Regional Government of Madrid, the European Union and the Josef Steiner Cancer Research Award 2003.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
DATABASES
Entrez gene
Omim
Ataxia-telangiectasia-like disorder
Xeroderma pigmentosum syndrome
SwissProt
FURTHER INFORMATION
Scientific progammes at the Centro Nacional de Investigaciones Oncológicas
Telomeres Information Center web site
Glossary
- NUCLEOSOME
-
The structure responsible in part for the compactness of a chromosome. Each nucleosome consists of a sequence of DNA wrapped around a histone core.
- HETEROCHROMATIN
-
Chromosomal material that is tightly coiled and inactive in terms of gene expression.
- SATELLITE REPEATS
-
DNA that contains many tandem repeats of a short basic repeating unit. Both the major and minor satellite repeats are located at pericentric heterochromatin.
- RETINOBLASTOMA PROTEINS
-
These are a family of tumour supressor proteins that share a similar structure and function. Mutations in RB1 have been associated with retinoblastoma, a malignant eye tumour in children.
- RETT SYNDROME
-
An X-linked dominant neurological disorder that affects girls only and is one of the most common causes of mental retardation in females. Rett syndrome is due to a mutation in the MECP2 gene (methyl-CpG-binding protein 2).
- ANTICIPATION
-
A phenomenon in which a genetic disease appears earlier in the lifetime of an individual with each successive generation.
- MITOTIC CATASTROPHE
-
The loss of cell viability that results from the attempted aberrant chromosome segregation in the presence of severe cellular damage (for example, short telomeres).
- snoRNA
-
Small nucleolar RNAs. The functions of these snoRNAs include RNA cleavage reactions, as well as specifying sites of ribose methylation and pseudouridylation. Mutations in DKC1 (dyskeratosis congenita 1, dyskerin) result in defective pseudouridylation of the H/ACA box class of snoRNAs.
Rights and permissions
About this article
Cite this article
Blasco, M. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 6, 611–622 (2005). https://doi.org/10.1038/nrg1656
Issue Date:
DOI: https://doi.org/10.1038/nrg1656
This article is cited by
-
Telomere length as biomarker of nutritional therapy for prevention of type 2 diabetes mellitus development in patients with coronary heart disease: CORDIOPREV randomised controlled trial
Cardiovascular Diabetology (2024)
-
T-cell lymphocytes’ aging clock: telomeres, telomerase and aging
Biogerontology (2024)
-
The Social Environment Matters for Telomere Length and Internalizing Problems During Adolescence
Journal of Youth and Adolescence (2024)
-
Effects of hTERT transfection on the telomere and telomerase of Periplaneta americana cells in vitro
AMB Express (2023)
-
MCCC2 is a novel mediator between mitochondria and telomere and functions as an oncogene in colorectal cancer
Cellular & Molecular Biology Letters (2023)