Interaction of voltage-gated sodium channels with the extracellular matrix molecules tenascin-C and tenascin-R
- PMID: 9861042
- PMCID: PMC28116
- DOI: 10.1073/pnas.95.26.15753
Interaction of voltage-gated sodium channels with the extracellular matrix molecules tenascin-C and tenascin-R
Abstract
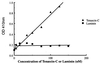
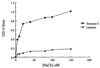
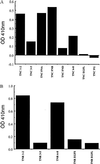
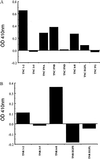
The type IIA rat brain sodium channel is composed of three subunits: a large pore-forming alpha subunit and two smaller auxiliary subunits, beta1 and beta2. The beta subunits are single membrane-spanning glycoproteins with one Ig-like motif in their extracellular domains. The Ig motif of the beta2 subunit has close structural similarity to one of the six Ig motifs in the extracellular domain of the cell adhesion molecule contactin (also called F3 or F11), which binds to the extracellular matrix molecules tenascin-C and tenascin-R. We investigated the binding of the purified sodium channel and the extracellular domain of the beta2 subunit to tenascin-C and tenascin-R in vitro. Incubation of purified sodium channels on microtiter plates coated with tenascin-C revealed saturable and specific binding with an apparent Kd of approximately 15 nM. Glutathione S-transferase-tagged fusion proteins containing various segments of tenascin-C and tenascin-R were purified, digested with thrombin to remove the epitope tag, immobilized on microtiter dishes, and tested for their ability to bind purified sodium channel or the epitope-tagged extracellular domain of beta2 subunits. Both purified sodium channels and the extracellular domain of the beta2 subunit bound specifically to fibronectin type III repeats 1-2, A, B, and 6-8 of tenascin-C and fibronectin type III repeats 1-2 and 6-8 of tenascin-R but not to the epidermal growth factor-like domain or the fibrinogen-like domain of these molecules. The binding of neuronal sodium channels to extracellular matrix molecules such as tenascin-C and tenascin-R may play a crucial role in localizing sodium channels in high density at axon initial segments and nodes of Ranvier or in regulating the activity of immobilized sodium channels in these locations.
Figures
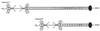
Similar articles
-
Tenascin-R is a functional modulator of sodium channel beta subunits.J Biol Chem. 1999 Sep 10;274(37):26511-7. doi: 10.1074/jbc.274.37.26511. J Biol Chem. 1999. PMID: 10473612
-
Sodium channel beta subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell-cell contact.J Biol Chem. 2000 Apr 14;275(15):11383-8. doi: 10.1074/jbc.275.15.11383. J Biol Chem. 2000. PMID: 10753953
-
Heterophilic interactions of sodium channel beta1 subunits with axonal and glial cell adhesion molecules.J Biol Chem. 2004 Dec 10;279(50):52744-52. doi: 10.1074/jbc.M405990200. Epub 2004 Oct 4. J Biol Chem. 2004. PMID: 15466474
-
Molecular properties of brain sodium channels: an important target for anticonvulsant drugs.Adv Neurol. 1999;79:441-56. Adv Neurol. 1999. PMID: 10514834 Review.
-
The role of sodium channels in cell adhesion.Front Biosci. 2002 Jan 1;7:12-23. doi: 10.2741/isom. Front Biosci. 2002. PMID: 11779698 Review.
Cited by
-
Decreased ability to manage increases in reactive oxygen species may underlie susceptibility to arrhythmias in mice lacking Scn1b.Am J Physiol Heart Circ Physiol. 2024 Oct 1;327(4):H723-H732. doi: 10.1152/ajpheart.00265.2024. Epub 2024 Aug 9. Am J Physiol Heart Circ Physiol. 2024. PMID: 39120465
-
Navβ2 Intracellular Fragments Contribute to Aβ1-42-Induced Cognitive Impairment and Synaptic Deficit Through Transcriptional Suppression of BDNF.Mol Neurobiol. 2024 Jul 5. doi: 10.1007/s12035-024-04317-y. Online ahead of print. Mol Neurobiol. 2024. PMID: 38965172
-
Voltage-gated sodium channels: from roles and mechanisms in the metastatic cell behavior to clinical potential as therapeutic targets.Front Pharmacol. 2023 Jun 30;14:1206136. doi: 10.3389/fphar.2023.1206136. eCollection 2023. Front Pharmacol. 2023. PMID: 37456756 Free PMC article. Review.
-
Single-cell transcriptomic landscape of the developing human spinal cord.Nat Neurosci. 2023 May;26(5):902-914. doi: 10.1038/s41593-023-01311-w. Epub 2023 Apr 24. Nat Neurosci. 2023. PMID: 37095394
-
Responses in fast-spiking interneuron firing rates to parameter variations associated with degradation of perineuronal nets.J Comput Neurosci. 2023 May;51(2):283-298. doi: 10.1007/s10827-023-00849-9. Epub 2023 Apr 14. J Comput Neurosci. 2023. PMID: 37058180 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous


