Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites
- PMID: 9670049
- PMCID: PMC2212459
- DOI: 10.1084/jem.188.2.373
Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites
Abstract
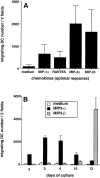
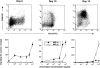
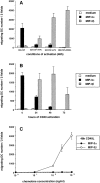
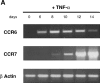
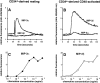
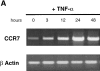
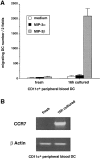
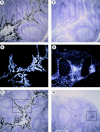
DCs (dendritic cells) function as sentinels of the immune system. They traffic from the blood to the tissues where, while immature, they capture antigens. They then leave the tissues and move to the draining lymphoid organs where, converted into mature DC, they prime naive T cells. This suggestive link between DC traffic pattern and functions led us to investigate the chemokine responsiveness of DCs during their development and maturation. DCs were differentiated either from CD34(+) hematopoietic progenitor cells (HPCs) cultured with granulocyte/macrophage colony-stimulating factor (GM-CSF) plus tumor necrosis factor (TNF)-alpha or from monocytes cultured with GM-CSF plus interleukin 4. Immature DCs derived from CD34(+) HPCs migrate most vigorously in response to macrophage inflammatory protein (MIP)-3alpha, but also to MIP-1alpha and RANTES (regulated on activation, normal T cell expressed and secreted). Upon maturation, induced by either TNF-alpha, lipopolysaccharide, or CD40L, DCs lose their response to these three chemokines when they acquire a sustained responsiveness to a single other chemokine, MIP-3beta. CC chemokine receptor (CCR)6 and CCR7 are the only known receptors for MIP-3alpha and MIP-3beta, respectively. The observation that CCR6 mRNA expression decreases progressively as DCs mature, whereas CCR7 mRNA expression is sharply upregulated, provides a likely explanation for the changes in chemokine responsiveness. Similarly, MIP-3beta responsiveness and CCR7 expression are induced upon maturation of monocyte- derived DCs. Furthermore, the chemotactic response to MIP-3beta is also acquired by CD11c+ DCs isolated from blood after spontaneous maturation. Finally, detection by in situ hybridization of MIP-3alpha mRNA only within inflamed epithelial crypts of tonsils, and of MIP-3beta mRNA specifically in T cell-rich areas, suggests a role for MIP-3alpha/CCR6 in recruitment of immature DCs at site of injury and for MIP-3beta/CCR7 in accumulation of antigen-loaded mature DCs in T cell-rich areas.
Figures
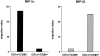

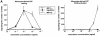
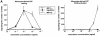


Similar articles
-
Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine.J Exp Med. 2000 Apr 17;191(8):1381-94. doi: 10.1084/jem.191.8.1381. J Exp Med. 2000. PMID: 10770804 Free PMC article.
-
Regulation of dendritic cell trafficking: a process that involves the participation of selective chemokines.J Leukoc Biol. 1999 Aug;66(2):252-62. doi: 10.1002/jlb.66.2.252. J Leukoc Biol. 1999. PMID: 10449163 Review.
-
Dendritic cell biology and regulation of dendritic cell trafficking by chemokines.Springer Semin Immunopathol. 2000;22(4):345-69. doi: 10.1007/s002810000053. Springer Semin Immunopathol. 2000. PMID: 11155441 Review.
-
Macrophage inflammatory protein 3alpha is involved in the constitutive trafficking of epidermal langerhans cells.J Exp Med. 1999 Dec 20;190(12):1755-68. doi: 10.1084/jem.190.12.1755. J Exp Med. 1999. PMID: 10601351 Free PMC article.
-
Differential responsiveness to constitutive vs. inducible chemokines of immature and mature mouse dendritic cells.J Leukoc Biol. 1999 Sep;66(3):489-94. doi: 10.1002/jlb.66.3.489. J Leukoc Biol. 1999. PMID: 10496320
Cited by
-
CCL3 Promotes Cutaneous Wound Healing Through Recruiting Macrophages in Mice.Cell Transplant. 2024 Jan-Dec;33:9636897241264912. doi: 10.1177/09636897241264912. Cell Transplant. 2024. PMID: 39076075 Free PMC article.
-
Challenges and Future Perspectives in Modeling Neurodegenerative Diseases Using Organ-on-a-Chip Technology.Adv Sci (Weinh). 2024 Aug;11(32):e2403892. doi: 10.1002/advs.202403892. Epub 2024 Jun 23. Adv Sci (Weinh). 2024. PMID: 38922799 Free PMC article. Review.
-
On-Site Stimulation of Dendritic Cells by Cancer-Derived Extracellular Vesicles on a Core-Shell Nanowire Platform.ACS Appl Mater Interfaces. 2024 Jun 12;16(23):29570-29580. doi: 10.1021/acsami.4c00283. Epub 2024 May 28. ACS Appl Mater Interfaces. 2024. PMID: 38804616 Free PMC article.
-
The Impact of Phenotype of Inflammatory Bowel Diseases, Inflammation Activity and Therapy on Mucosal Mature Cd83+ Dendritic Cell.J Clin Med. 2024 Apr 3;13(7):2070. doi: 10.3390/jcm13072070. J Clin Med. 2024. PMID: 38610835 Free PMC article.
-
Control of adaptive immunity by pattern recognition receptors.Immunity. 2024 Apr 9;57(4):632-648. doi: 10.1016/j.immuni.2024.03.014. Immunity. 2024. PMID: 38599163 Review.
References
-
- Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. - PubMed
-
- Kaplan G, Walsh G, Guido LS, Meyn P, Burkhardt RA, Abalos RM, Barker J, Frindt PA, Fajardo TT, Celona R, Cohn ZA. Novel responses of human skin to intradermal recombinant granulocyte/macrophage-colony–stimulating factor: Langerhans cell recruitment, keratinocyte growth, and enhanced wound healing. J Exp Med. 1992;175:1717–1728. - PMC - PubMed
-
- Streilein JW, Grammer SF. In vitro evidence that Langerhans cells can adopt two functionally distinct forms capable of antigen presentation to T lymphocytes. J Immunol. 1989;143:3925–3933. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials


