A simplified system for generating recombinant adenoviruses
- PMID: 9482916
- PMCID: PMC19394
- DOI: 10.1073/pnas.95.5.2509
A simplified system for generating recombinant adenoviruses
Abstract
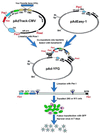
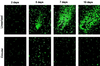
Recombinant adenoviruses provide a versatile system for gene expression studies and therapeutic applications. We report herein a strategy that simplifies the generation and production of such viruses. A recombinant adenoviral plasmid is generated with a minimum of enzymatic manipulations, using homologous recombination in bacteria rather than in eukaryotic cells. After transfections of such plasmids into a mammalian packaging cell line, viral production is conveniently followed with the aid of green fluorescent protein, encoded by a gene incorporated into the viral backbone. Homogeneous viruses can be obtained from this procedure without plaque purification. This system should expedite the process of generating and testing recombinant adenoviruses for a variety of purposes.
Figures



Similar articles
-
A protocol for rapid generation of recombinant adenoviruses using the AdEasy system.Nat Protoc. 2007;2(5):1236-47. doi: 10.1038/nprot.2007.135. Nat Protoc. 2007. PMID: 17546019
-
Generation of recombinant adenovirus using the Escherichia coli BJ5183 recombination system.Methods Mol Med. 2007;130:61-8. doi: 10.1385/1-59745-166-5:61. Methods Mol Med. 2007. PMID: 17401164
-
Novel cloning method for recombinant adenovirus construction in Escherichia coli.Biotechniques. 1999 Mar;26(3):502-8. doi: 10.2144/99263rr01. Biotechniques. 1999. PMID: 10090992
-
Delivery of bacterial artificial chromosomes into mammalian cells with psoralen-inactivated adenovirus carrier.Nucleic Acids Res. 1997 May 15;25(10):1950-6. doi: 10.1093/nar/25.10.1950. Nucleic Acids Res. 1997. PMID: 9115362 Free PMC article.
-
The uses of green fluorescent protein in mammalian cells.Methods Biochem Anal. 2006;47:305-37. doi: 10.1002/0471739499.ch14. Methods Biochem Anal. 2006. PMID: 16335719 Review. No abstract available.
Cited by
-
FastAd: A versatile toolkit for rapid generation of single adenoviruses or diverse adenoviral vector libraries.Mol Ther Methods Clin Dev. 2024 Oct 18;32(4):101356. doi: 10.1016/j.omtm.2024.101356. eCollection 2024 Dec 12. Mol Ther Methods Clin Dev. 2024. PMID: 39559559 Free PMC article.
-
Temporal regulation of acetylation status determines PARP1 role in DNA damage response and metabolic homeostasis.Sci Adv. 2024 Oct 18;10(42):eado7720. doi: 10.1126/sciadv.ado7720. Epub 2024 Oct 18. Sci Adv. 2024. PMID: 39423262 Free PMC article.
-
DDIT3 switches osteogenic potential of BMP9 to lipogenic by attenuating Wnt/β-catenin signaling via up-regulating DKK1 in mesenchymal stem cells.Aging (Albany NY). 2024 Sep 26;16(18):12543-12558. doi: 10.18632/aging.206091. Epub 2024 Sep 26. Aging (Albany NY). 2024. PMID: 39331002 Free PMC article.
-
A Simple and Versatile Method for Ex Vivo Monitoring of Goat Vaginal Mucosa Transduction by Viral Vector Vaccines.Vaccines (Basel). 2024 Jul 29;12(8):851. doi: 10.3390/vaccines12080851. Vaccines (Basel). 2024. PMID: 39203977 Free PMC article.
-
GAPDH suppresses adenovirus-induced oxidative stress and enables a superfast production of recombinant adenovirus.Genes Dis. 2024 May 31;11(6):101344. doi: 10.1016/j.gendis.2024.101344. eCollection 2024 Nov. Genes Dis. 2024. PMID: 39188753 Free PMC article.
References
-
- Miller A D. Nature (London) 1992;357:455–460. - PubMed
-
- Morgan R A, Anderson F A. Annu Rev Biochem. 1993;62:191–217. - PubMed
-
- Graham F L, Prevec L. Methods Mol Biol. 1991;7:109–128. - PubMed
-
- Berkner K L. BioTechniques. 1988;6:616–629. - PubMed
-
- Shenk T. In: Fields Virology. Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Philadelphia: Lippincott; 1996. pp. 2111–2148.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials


