On the structural basis for size-selective permeation of organic cations through the voltage-gated sodium channel. Effect of alanine mutations at the DEKA locus on selectivity, inhibition by Ca2+ and H+, and molecular sieving
- PMID: 9382897
- PMCID: PMC2229404
- DOI: 10.1085/jgp.110.6.693
On the structural basis for size-selective permeation of organic cations through the voltage-gated sodium channel. Effect of alanine mutations at the DEKA locus on selectivity, inhibition by Ca2+ and H+, and molecular sieving
Abstract
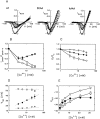
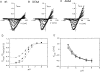
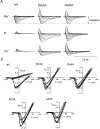
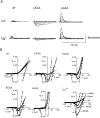
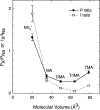
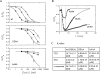
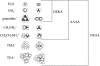
Recent evidence indicates that ionic selectivity in voltage-gated Na+ channels is mediated by a small number of residues in P-region segments that link transmembrane elements S5 and S6 in each of four homologous domains denoted I, II, III, and IV. Important determinants for this function appear to be a set of conserved charged residues in the first three homologous domains, Asp(I), Glu(II), and Lys(III), located in a region of the pore called the DEKA locus. In this study, we examined several Ala-substitution mutations of these residues for alterations in ionic selectivity, inhibition of macroscopic current by external Ca2+ and H+, and molecular sieving behavior using a series of organic cations ranging in size from ammonium to tetraethylammonium. Whole-cell recording of wild-type and mutant channels of the rat muscle micro1 Na+ channel stably expressed in HEK293 cells was used to compare macroscopic current-voltage behavior in the presence of various external cations and an intracellular reference solution containing Cs+ and very low Ca2+. In particular, we tested the hypothesis that the Lys residue in domain III of the DEKA locus is responsible for restricting the permeation of large organic cations. Mutation of Lys(III) to Ala largely eliminated selectivity among the group IA monovalent alkali cations (Li+, Na+, K+, Rb+, Cs+) and permitted inward current of group IIA divalent cations (Mg2+, Ca2+, Sr2+, Ba2+). This same mutation also resulted in the acquisition of permeability to many large organic cations such as methylammonium, tetramethylammonium, and tetraethylammonium, all of which are impermeant in the native channel. The results lead to the conclusion that charged residues of the DEKA locus play an important role in molecular sieving behavior of the Na+ channel pore, a function that has been previously attributed to a hypothetical region of the channel called the "selectivity filter." A detailed examination of individual contributions of the Asp(I), Glu(II), and Lys(III) residues and the dependence on molecular size suggests that relative permeability of organic cations is a complex function of the size, charge, and polarity of these residues and cation substrates. As judged by effects on macroscopic conductance, charged residues of the DEKA locus also appear to play a role in the mechanisms of block by external Ca2+ and H+, but are not essential for the positive shift in activation voltage that is produced by these ions.
Figures




Similar articles
-
Permeation of large tetra-alkylammonium cations through mutant and wild-type voltage-gated sodium channels as revealed by relief of block at high voltage.J Gen Physiol. 2000 Apr;115(4):435-54. doi: 10.1085/jgp.115.4.435. J Gen Physiol. 2000. PMID: 10736311 Free PMC article.
-
Altered ionic selectivity of the sodium channel revealed by cysteine mutations within the pore.J Gen Physiol. 1997 Apr;109(4):463-75. doi: 10.1085/jgp.109.4.463. J Gen Physiol. 1997. PMID: 9101405 Free PMC article.
-
Pore properties of rat brain II sodium channels mutated in the selectivity filter domain.Eur Biophys J. 1996;25(2):75-91. doi: 10.1007/s002490050020. Eur Biophys J. 1996. PMID: 9035373
-
Molecular pore structure of voltage-gated sodium and calcium channels.Braz J Med Biol Res. 1994 Dec;27(12):2781-802. Braz J Med Biol Res. 1994. PMID: 7550000 Review.
-
The tetrodotoxin binding site is within the outer vestibule of the sodium channel.Mar Drugs. 2010 Feb 1;8(2):219-34. doi: 10.3390/md8020219. Mar Drugs. 2010. PMID: 20390102 Free PMC article. Review.
Cited by
-
Conotoxins Targeting Voltage-Gated Sodium Ion Channels.Pharmacol Rev. 2024 Aug 15;76(5):828-845. doi: 10.1124/pharmrev.123.000923. Pharmacol Rev. 2024. PMID: 38914468 Review.
-
A structural atlas of druggable sites on Nav channels.Channels (Austin). 2024 Dec;18(1):2287832. doi: 10.1080/19336950.2023.2287832. Epub 2023 Nov 30. Channels (Austin). 2024. PMID: 38033122 Free PMC article. Review.
-
Vicia faba SV channel VfTPC1 is a hyperexcitable variant of plant vacuole Two Pore Channels.Elife. 2023 Nov 22;12:e86384. doi: 10.7554/eLife.86384. Elife. 2023. PMID: 37991833 Free PMC article.
-
Structure of human NaV1.6 channel reveals Na+ selectivity and pore blockade by 4,9-anhydro-tetrodotoxin.Nat Commun. 2023 Feb 23;14(1):1030. doi: 10.1038/s41467-023-36766-9. Nat Commun. 2023. PMID: 36823201 Free PMC article.
-
In Silico Analysis of Tetrodotoxin Binding in Voltage-Gated Sodium Ion Channels from Toxin-Resistant Animal Lineages.Mar Drugs. 2022 Nov 18;20(11):723. doi: 10.3390/md20110723. Mar Drugs. 2022. PMID: 36422001 Free PMC article.
References
-
- Armstrong CM, Gilly WF. Access resistance and space clamp problems associated with whole-cell patch clamping. Methods Enzymol. 1992;207:100–122. - PubMed
-
- Becker S, Prusak-Sochaczewski E, Zamponi G, Beck-Sickinger AG, Gordon RD, French RJ. Action of derivatives of μ-conotoxin GIIIA on sodium channels. Single amino acid substitutions in the toxin separately affect association and dissociation rates. Biochemistry. 1992;31:8229–8238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous


