Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in beta-catenin that are modulated by the Wnt signaling pathway
- PMID: 9060476
- PMCID: PMC2132470
- DOI: 10.1083/jcb.136.5.1123
Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in beta-catenin that are modulated by the Wnt signaling pathway
Abstract
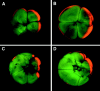
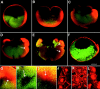
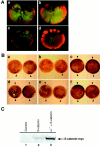
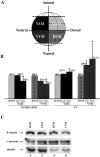
Eggs of Xenopus laevis undergo a postfertilization cortical rotation that specifies the position of the dorso-ventral axis and activates a transplantable dorsal-determining activity in dorsal blastomeres by the 32-cell stage. There have heretofore been no reported dorso-ventral asymmetries in endogenous signaling proteins that may be involved in this dorsal-determining activity during early cleavage stages. We focused on beta-catenin as a candidate for an asymmetrically localized dorsal-determining factor since it is both necessary and sufficient for dorsal axis formation. We report that beta-catenin displays greater cytoplasmic accumulation on the future dorsal side of the Xenopus embryo by the two-cell stage. This asymmetry persists and increases through early cleavage stages, with beta-catenin accumulating in dorsal but not ventral nuclei by the 16- to 32-cell stages. We then investigated which potential signaling factors and pathways are capable of modulating the steady-state levels of endogenous beta-catenin. Steady-state levels and nuclear accumulation of beta-catenin increased in response to ectopic Xenopus Wnt-8 (Xwnt-8) and to the inhibition of glycogen synthase kinase-3, whereas neither Xwnt-5A, BVg1, nor noggin increased beta-catenin levels before the mid-blastula stage. As greater levels and nuclear accumulation of beta-catenin on the future dorsal side of the embryo correlate with the induction of specific dorsal genes, our data suggest that early asymmetries in beta-catenin presage and may specify dorso-ventral differences in gene expression and cell fate. Our data further support the hypothesis that these dorso-ventral differences in beta-catenin arise in response to the postfertilization activation of a signaling pathway that involves Xenopus glycogen synthase kinase-3.
Figures



Similar articles
-
Heads or tails? Amphioxus and the evolution of anterior-posterior patterning in deuterostomes.Dev Biol. 2002 Jan 15;241(2):209-28. doi: 10.1006/dbio.2001.0503. Dev Biol. 2002. PMID: 11784106 Review.
-
Conservation of intracellular Wnt signaling components in dorsal-ventral axis formation in zebrafish.Dev Genes Evol. 1999 Jan;209(1):48-58. doi: 10.1007/s004270050226. Dev Genes Evol. 1999. PMID: 9914418
-
Interaction among GSK-3, GBP, axin, and APC in Xenopus axis specification.J Cell Biol. 2000 Feb 21;148(4):691-702. doi: 10.1083/jcb.148.4.691. J Cell Biol. 2000. PMID: 10684251 Free PMC article.
-
Relationship of vegetal cortical dorsal factors in the Xenopus egg with the Wnt/beta-catenin signaling pathway.Mech Dev. 1999 Dec;89(1-2):93-102. doi: 10.1016/s0925-4773(99)00210-5. Mech Dev. 1999. PMID: 10559484
-
New steps in the Wnt/beta-catenin signal transduction pathway.Recent Prog Horm Res. 2000;55:225-36. Recent Prog Horm Res. 2000. PMID: 11036939 Review.
Cited by
-
The pericardium forms as a distinct structure during heart formation.bioRxiv [Preprint]. 2024 Sep 28:2024.09.18.613484. doi: 10.1101/2024.09.18.613484. bioRxiv. 2024. PMID: 39345600 Free PMC article. Preprint.
-
Differentiation of Pluripotent Stem Cells for Disease Modeling: Learning from Heart Development.Pharmaceuticals (Basel). 2024 Mar 5;17(3):337. doi: 10.3390/ph17030337. Pharmaceuticals (Basel). 2024. PMID: 38543122 Free PMC article. Review.
-
Determining zebrafish dorsal organizer size by a negative feedback loop between canonical/non-canonical Wnts and Tlr4/NFκB.Nat Commun. 2023 Nov 8;14(1):7194. doi: 10.1038/s41467-023-42963-3. Nat Commun. 2023. PMID: 37938219 Free PMC article.
-
β-Catenin and SOX2 Interaction Regulate Visual Experience-Dependent Cell Homeostasis in the Developing Xenopus Thalamus.Int J Mol Sci. 2023 Sep 2;24(17):13593. doi: 10.3390/ijms241713593. Int J Mol Sci. 2023. PMID: 37686400 Free PMC article.
-
Lysosomes are required for early dorsal signaling in the Xenopus embryo.Proc Natl Acad Sci U S A. 2022 Apr 26;119(17):e2201008119. doi: 10.1073/pnas.2201008119. Epub 2022 Apr 21. Proc Natl Acad Sci U S A. 2022. PMID: 35446621 Free PMC article.
References
-
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature (Lond) 1996;382:638–642. - PubMed
-
- Bhanot P, Brink M, Samos CH, Hsieh J-C, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophilafunctions as a Wingless receptor. Nature (Lond) 1996;382:225–230. - PubMed
-
- Brannon M, Kimelman D. Activation of Siamoisby the Wnt pathway. Dev Biol. 1996;180:344–347. - PubMed
-
- Cardellini P. Reversal of dorsal ventral polarity in Xenopus laevisembryos by 180° rotation of the animal micromeres at the eight cell stage. Dev Biol. 1988;128:428–434. - PubMed
-
- Carnac G, Kodjabachian L, Gurdon JB, Lemaire P. The homeobox gene Siamoisis a target of the Wnt dorsalization pathway and triggers organizer activity in the absence of mesoderm. Development (Camb) 1996;122:2055–3065. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials


