Haltere afferents provide direct, electrotonic input to a steering motor neuron in the blowfly, Calliphora
- PMID: 8756451
- PMCID: PMC6579303
- DOI: 10.1523/JNEUROSCI.16-16-05225.1996
Haltere afferents provide direct, electrotonic input to a steering motor neuron in the blowfly, Calliphora
Abstract
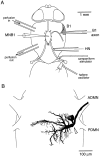
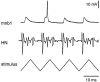
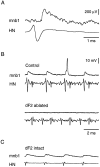
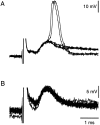
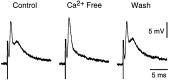
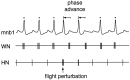
The first basalar muscle (b1) is one of 17 small muscles in flies that control changes in wing stroke kinematics during steering maneuvers. The b1 is unique, however, in that it fires a single phase-locked spike during each wingbeat cycle. The phaselocked firing of the b1's motor neuron (mnb1) is thought to result from wingbeat-synchronous mechanosensory input, such as that originating from the campaniform sensilla at the base of the halteres. Halteres are sophisticated equilibrium organs of flies that function to detect angular rotations of the body during flight. We have developed a new preparation to determine whether the campaniform sensilla at the base of the halteres are responsible for the phasic activity of b1. Using intracellular recording and mechanical stimulation, we have found one identified haltere campaniform field (dF2) that provides strong synaptic input to the mnb1. This haltere to mnb1 connection consists of a fast and a slow component. The fast component is monosynaptic, mediated by an electrical synapse, and thus can follow haltere stimulation at high frequencies. The slow component is possibly polysynaptic, mediated by a chemical synapse, and fatigues at high stimulus frequencies. Thus, the fast monosynaptic electrical pathway between haltere afferents and mnb1 may be responsible in part for the phase-locked firing of b1 during flight.
Figures
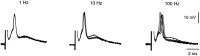
Similar articles
-
Flies tune the activity of their multifunctional gyroscope.Curr Biol. 2024 Aug 19;34(16):3644-3653.e3. doi: 10.1016/j.cub.2024.06.066. Epub 2024 Jul 24. Curr Biol. 2024. PMID: 39053466
-
Convergent mechanosensory input structures the firing phase of a steering motor neuron in the blowfly, Calliphora.J Neurophysiol. 1999 Oct;82(4):1916-26. doi: 10.1152/jn.1999.82.4.1916. J Neurophysiol. 1999. PMID: 10515981
-
Position-specific central projections of mechanosensory neurons on the haltere of the blow fly, Calliphora vicina.J Comp Neurol. 1996 Jun 3;369(3):405-18. doi: 10.1002/(SICI)1096-9861(19960603)369:3<405::AID-CNE6>3.0.CO;2-9. J Comp Neurol. 1996. PMID: 8743421
-
Visual input to the efferent control system of a fly's "gyroscope".Science. 1998 Apr 10;280(5361):289-92. doi: 10.1126/science.280.5361.289. Science. 1998. PMID: 9535659
-
Multisensory systems integration for high-performance motor control in flies.Curr Opin Neurobiol. 2010 Jun;20(3):347-52. doi: 10.1016/j.conb.2010.02.002. Epub 2010 Mar 2. Curr Opin Neurobiol. 2010. PMID: 20202821 Free PMC article. Review.
Cited by
-
Flies tune the activity of their multifunctional gyroscope.Curr Biol. 2024 Aug 19;34(16):3644-3653.e3. doi: 10.1016/j.cub.2024.06.066. Epub 2024 Jul 24. Curr Biol. 2024. PMID: 39053466
-
Synaptic architecture of leg and wing premotor control networks in Drosophila.Nature. 2024 Jul;631(8020):369-377. doi: 10.1038/s41586-024-07600-z. Epub 2024 Jun 26. Nature. 2024. PMID: 38926579
-
Asynchronous haltere input drives specific wing and head movements in Drosophila.Proc Biol Sci. 2024 Jun;291(2024):20240311. doi: 10.1098/rspb.2024.0311. Epub 2024 Jun 12. Proc Biol Sci. 2024. PMID: 38864337
-
Intraspecific Variation in the Placement of Campaniform Sensilla on the Wings of the Hawkmoth Manduca Sexta.Integr Org Biol. 2024 Mar 13;6(1):obae007. doi: 10.1093/iob/obae007. eCollection 2024. Integr Org Biol. 2024. PMID: 38715720 Free PMC article.
-
An information theoretic method to resolve millisecond-scale spike timing precision in a comprehensive motor program.PLoS Comput Biol. 2023 Jun 12;19(6):e1011170. doi: 10.1371/journal.pcbi.1011170. eCollection 2023 Jun. PLoS Comput Biol. 2023. PMID: 37307288 Free PMC article.
References
-
- Berry MS, Pentreath VW. Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res. 1976;105:1–20. - PubMed
-
- Bonhag PE (1949) The thoracic mechanism of the adult horsefly (Diptera: Tabanidae). Mem Cornell Univ Agric Exp Stat 285.
-
- Carpenter RHS. Pion; London: 1988. Movements of the eyes. .
-
- Chan WP, Dickinson MH. Position-specific central projections of mechanosensory neurons on the haltere of the blow fly, Calliphora vicina . J Comp Neurol. 1996;369:405–418. - PubMed
-
- Cole ES, Palka J. The pattern of campaniform sensilla on the wing and haltere of Drosophila melanogaster and several of its homeotic mutants. J Embryol Exp Morphol. 1982;71:41–61. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

