Glioblastoma Tumor Microenvironment: An Important Modulator for Tumoral Progression and Therapy Resistance
- PMID: 39329940
- PMCID: PMC11430601
- DOI: 10.3390/cimb46090588
Glioblastoma Tumor Microenvironment: An Important Modulator for Tumoral Progression and Therapy Resistance
Abstract
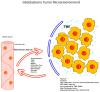
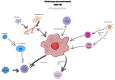
The race to find an effective treatment for glioblastoma (GBM) remains a critical topic, because of its high aggressivity and impact on survival and the quality of life. Currently, due to GBM's high heterogeneity, the conventional treatment success rate and response to therapy are relatively low, with a median survival rate of less than 20 months. A new point of view can be provided by the comprehension of the tumor microenvironment (TME) in pursuance of the development of new therapeutic strategies to aim for a longer survival rate with an improved quality of life and longer disease-free interval (DFI). The main components of the GBM TME are represented by the extracellular matrix (ECM), glioma cells and glioma stem cells (GSCs), immune cells (microglia, macrophages, neutrophils, lymphocytes), neuronal cells, all of them having dynamic interactions and being able to influence the tumoral growth, progression, and drug resistance thus being a potential therapeutic target. This paper will review the latest research on the GBM TME and the potential therapeutic targets to form an up-to-date strategy.
Keywords: extracellular matrix; glioblastoma; molecular diagnostic; tumor microenvironment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Decipher the Glioblastoma Microenvironment: The First Milestone for New Groundbreaking Therapeutic Strategies.Genes (Basel). 2021 Mar 20;12(3):445. doi: 10.3390/genes12030445. Genes (Basel). 2021. PMID: 33804731 Free PMC article. Review.
-
Tumor-associated microglia and macrophages in glioblastoma: From basic insights to therapeutic opportunities.Front Immunol. 2022 Jul 27;13:964898. doi: 10.3389/fimmu.2022.964898. eCollection 2022. Front Immunol. 2022. PMID: 35967394 Free PMC article. Review.
-
Glioblastoma Microenvironment and Invasiveness: New Insights and Therapeutic Targets.Int J Mol Sci. 2023 Apr 11;24(8):7047. doi: 10.3390/ijms24087047. Int J Mol Sci. 2023. PMID: 37108208 Free PMC article. Review.
-
Glioblastoma-instructed microglia transition to heterogeneous phenotypic states with phagocytic and dendritic cell-like features in patient tumors and patient-derived orthotopic xenografts.Genome Med. 2024 Apr 2;16(1):51. doi: 10.1186/s13073-024-01321-8. Genome Med. 2024. PMID: 38566128 Free PMC article.
-
Clinical Theranostics Trademark of Exosome in Glioblastoma Metastasis.ACS Biomater Sci Eng. 2023 Sep 11;9(9):5205-5221. doi: 10.1021/acsbiomaterials.3c00212. Epub 2023 Aug 14. ACS Biomater Sci Eng. 2023. PMID: 37578350 Review.
References
-
- Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. - DOI - PMC - PubMed
-
- Park Y.W., Vollmuth P., Foltyn-Dumitru M., Sahm F., Ahn S.S., Chang J.H., Kim S.H. The 2021 WHO Classification for Gliomas and Implications on Imaging Diagnosis: Part 1-Key Points of the Fifth Edition and Summary of Imaging Findings on Adult-Type Diffuse Gliomas. J. Magn. Reson. Imaging. 2023;58:677–689. doi: 10.1002/jmri.28743. - DOI - PubMed
-
- Stupp R., Taillibert S., Kanner A., Read W., Steinberg D., Lhermitte B., Toms S., Idbaih A., Ahluwalia M.S., Fink K., et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs. Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA. 2017;318:2306–2316. doi: 10.1001/jama.2017.18718. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources


