Capturing Patient Voice to Improve Outcomes That Matter to Patients with Desmoid Tumor
- PMID: 38863992
- PMCID: PMC11166168
- DOI: 10.2147/CMAR.S362694
Capturing Patient Voice to Improve Outcomes That Matter to Patients with Desmoid Tumor
Abstract
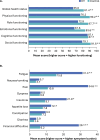
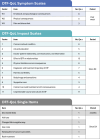
Desmoid tumors (DT) are rare, intermediate-grade sarcomas characterized by locally aggressive growths that commonly occur intra-abdominally, in the abdominal wall, or in the extremities. Desmoid tumors are 2-3-fold more common in females than males, with most patients aged <40 years at diagnosis. Clinical course of DT is highly variable but rarely fatal, with median overall survival >80% at 20 years. However, patient morbidity and DT symptom burden can be high. DT significantly reduce patient quality of life, imposing substantial physical, emotional, and social burdens. Pain, fatigue, and insomnia are common symptoms; disfigurement, mobility restrictions, and, rarely, the need for amputation may also result. Despite its limited impact on survival, patients with DT may have anxiety and depression levels commensurate with those associated with malignant sarcomas. Thus, DT impose an array of significant, long-term morbidities on a young patient population. In order to evaluate the impact of these morbidities, patient-reported outcome (PRO) tools are used, which assess outcomes of importance to patients that extend beyond traditional oncology endpoints. General or oncology-related PROs can be used; although currently, the only DT-specific, validated PRO measure is the GOunder/Desmoid Tumor Research Foundation DEsmoid Symptom/Impact Scale (GODDESS©), consisting of an 11-item DT Symptom Scale (DTSS) and a 17-item DT Impact Scale (DTIS). DTSS and DTIS were secondary endpoints in DeFi, a randomized phase 3 trial of nirogacestat; blinded, pooled data from DeFi were used to validate GODDESS reliability and responsiveness as a PRO measure in DT. Another DT-specific PRO measure, the Desmoid-Type Fibromatosis Quality of Life (DTF-QoL) questionnaire, has been developed but not validated. As novel DT therapies continue to be developed, incorporating DT-specific PRO measures into clinical trials will be key to capturing patient voice, improving outcomes of importance to this unique patient population, and assisting patients and providers in selecting optimal treatment.
Keywords: GODDESS; PRO; fibromatosis; patient-reported outcomes; quality of life; rare disease.
© 2024 Kasper et al.
Conflict of interest statement
Bernd Kasper: Consultant for Ayala, Bayer, Boehringer Ingelheim, GSK, Roche, and SpringWorks Therapeutics, Inc.; received honoraria from Bayer, GSK, and PharmaMar; research funding from Ayala, Cogent, PharmaMar, Rain Therapeutics, and SpringWorks Therapeutics, Inc. Mrinal Gounder: Personal honoraria/advisory boards and/or associated research paid to institution for Aadi, Ayala, Bayer, Boehringer Ingelheim, Daiichi, Epizyme, Karyopharm, Regeneron, Rain, SpringWorks Therapeutics, Inc., Tracon, and TYME; Other: Guidepoint, GLG, Third Bridge, and Flatiron Health; CME Honoraria: Medscape, More Health, Physicians Education Resource, MJ LifeSciences, and touchIME; Royalties: Wolters Kluwer, patents with MSKCC (GODDESS PRO), and uncompensated research with Foundation Medicine; Grants: Food and Drug Administration (R01 FD005105) and the National Cancer Institute, National Institutes of Health (P30CA008748)—core grant (CCSG shared resources and core facility). Lynne Hernandez: Executive Director of The Desmoid Tumor Research Foundation, which has received grants from SpringWorks Therapeutics, Inc., and Ayala Pharmaceuticals. Christina Baumgarten: Sarcoma Patients Advocacy Global Network received grants and sponsorship from SpringWorks Therapeutics, Inc. Ravin Ratan: Consultant for SpringWorks Therapeutics, Inc., Inhibrx, and Bayer. Research funding from SpringWorks Therapeutics, Inc., Ayala Pharmaceuticals, and C4 Therapeutics. The authors report no other conflicts of interest in this work.
Figures

Similar articles
-
GOunder/Desmoid Tumor Research Foundation DEsmoid Symptom/Impact Scale (GODDESS©): psychometric properties and clinically meaningful thresholds as assessed in the Phase 3 DeFi randomized controlled clinical trial.Qual Life Res. 2023 Oct;32(10):2861-2873. doi: 10.1007/s11136-023-03445-7. Epub 2023 Jun 22. Qual Life Res. 2023. PMID: 37347393 Free PMC article. Clinical Trial.
-
Prospective development of a patient-reported outcomes instrument for desmoid tumors or aggressive fibromatosis.Cancer. 2020 Feb 1;126(3):531-539. doi: 10.1002/cncr.32555. Epub 2019 Nov 6. Cancer. 2020. PMID: 31691276 Free PMC article.
-
Desmoid Tumors: A Comprehensive Review.Adv Ther. 2023 Sep;40(9):3697-3722. doi: 10.1007/s12325-023-02592-0. Epub 2023 Jul 12. Adv Ther. 2023. PMID: 37436594 Free PMC article. Review.
-
High prevalence of persistent emotional distress in desmoid tumor.Psychooncology. 2020 Feb;29(2):311-320. doi: 10.1002/pon.5250. Epub 2019 Dec 10. Psychooncology. 2020. PMID: 31778588
-
Current Management of Desmoid Tumors: A Review.JAMA Oncol. 2024 Aug 1;10(8):1121-1128. doi: 10.1001/jamaoncol.2024.1805. JAMA Oncol. 2024. PMID: 38900421 Review.
References
-
- Kasper B, Baumgarten C, Garcia J, et al. An update on the management of sporadic desmoid-type fibromatosis: a European Consensus Initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG). Ann Oncol. 2017;28(10):2399–2408. doi:10.1093/annonc/mdx323 - DOI - PMC - PubMed
-
- Constantinidou A, Judson I, Litchman C. Clinical presentation of desmoid tumor. In: Litchman C, editor. Desmoid Tumors. Springer; 2011:5–16.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous


