Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis
- PMID: 38174776
- PMCID: PMC10765473
- DOI: 10.1002/14651858.CD011381.pub3
Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis
Abstract
Background: Different therapeutic strategies are available for the treatment of people with relapsing-remitting multiple sclerosis (RRMS), including immunomodulators, immunosuppressants and biological agents. Although each one of these therapies reduces relapse frequency and slows disability accumulation compared to no treatment, their relative benefit remains unclear. This is an update of a Cochrane review published in 2015.
Objectives: To compare the efficacy and safety, through network meta-analysis, of interferon beta-1b, interferon beta-1a, glatiramer acetate, natalizumab, mitoxantrone, fingolimod, teriflunomide, dimethyl fumarate, alemtuzumab, pegylated interferon beta-1a, daclizumab, laquinimod, azathioprine, immunoglobulins, cladribine, cyclophosphamide, diroximel fumarate, fludarabine, interferon beta 1-a and beta 1-b, leflunomide, methotrexate, minocycline, mycophenolate mofetil, ofatumumab, ozanimod, ponesimod, rituximab, siponimod and steroids for the treatment of people with RRMS.
Search methods: CENTRAL, MEDLINE, Embase, and two trials registers were searched on 21 September 2021 together with reference checking, citation searching and contact with study authors to identify additional studies. A top-up search was conducted on 8 August 2022.
Selection criteria: Randomised controlled trials (RCTs) that studied one or more of the available immunomodulators and immunosuppressants as monotherapy in comparison to placebo or to another active agent, in adults with RRMS.
Data collection and analysis: Two authors independently selected studies and extracted data. We considered both direct and indirect evidence and performed data synthesis by pairwise and network meta-analysis. Certainty of the evidence was assessed by the GRADE approach.
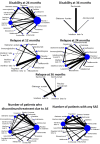
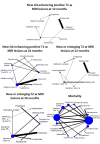
Main results: We included 50 studies involving 36,541 participants (68.6% female and 31.4% male). Median treatment duration was 24 months, and 25 (50%) studies were placebo-controlled. Considering the risk of bias, the most frequent concern was related to the role of the sponsor in the authorship of the study report or in data management and analysis, for which we judged 68% of the studies were at high risk of other bias. The other frequent concerns were performance bias (34% judged as having high risk) and attrition bias (32% judged as having high risk). Placebo was used as the common comparator for network analysis. Relapses over 12 months: data were provided in 18 studies (9310 participants). Natalizumab results in a large reduction of people with relapses at 12 months (RR 0.52, 95% CI 0.43 to 0.63; high-certainty evidence). Fingolimod (RR 0.48, 95% CI 0.39 to 0.57; moderate-certainty evidence), daclizumab (RR 0.55, 95% CI 0.42 to 0.73; moderate-certainty evidence), and immunoglobulins (RR 0.60, 95% CI 0.47 to 0.79; moderate-certainty evidence) probably result in a large reduction of people with relapses at 12 months. Relapses over 24 months: data were reported in 28 studies (19,869 participants). Cladribine (RR 0.53, 95% CI 0.44 to 0.64; high-certainty evidence), alemtuzumab (RR 0.57, 95% CI 0.47 to 0.68; high-certainty evidence) and natalizumab (RR 0.56, 95% CI 0.48 to 0.65; high-certainty evidence) result in a large decrease of people with relapses at 24 months. Fingolimod (RR 0.54, 95% CI 0.48 to 0.60; moderate-certainty evidence), dimethyl fumarate (RR 0.62, 95% CI 0.55 to 0.70; moderate-certainty evidence), and ponesimod (RR 0.58, 95% CI 0.48 to 0.70; moderate-certainty evidence) probably result in a large decrease of people with relapses at 24 months. Glatiramer acetate (RR 0.84, 95%, CI 0.76 to 0.93; moderate-certainty evidence) and interferon beta-1a (Avonex, Rebif) (RR 0.84, 95% CI 0.78 to 0.91; moderate-certainty evidence) probably moderately decrease people with relapses at 24 months. Relapses over 36 months findings were available from five studies (3087 participants). None of the treatments assessed showed moderate- or high-certainty evidence compared to placebo. Disability worsening over 24 months was assessed in 31 studies (24,303 participants). Natalizumab probably results in a large reduction of disability worsening (RR 0.59, 95% CI 0.46 to 0.75; moderate-certainty evidence) at 24 months. Disability worsening over 36 months was assessed in three studies (2684 participants) but none of the studies used placebo as the comparator. Treatment discontinuation due to adverse events data were available from 43 studies (35,410 participants). Alemtuzumab probably results in a slight reduction of treatment discontinuation due to adverse events (OR 0.39, 95% CI 0.19 to 0.79; moderate-certainty evidence). Daclizumab (OR 2.55, 95% CI 1.40 to 4.63; moderate-certainty evidence), fingolimod (OR 1.84, 95% CI 1.31 to 2.57; moderate-certainty evidence), teriflunomide (OR 1.82, 95% CI 1.19 to 2.79; moderate-certainty evidence), interferon beta-1a (OR 1.48, 95% CI 0.99 to 2.20; moderate-certainty evidence), laquinimod (OR 1.49, 95 % CI 1.00 to 2.15; moderate-certainty evidence), natalizumab (OR 1.57, 95% CI 0.81 to 3.05), and glatiramer acetate (OR 1.48, 95% CI 1.01 to 2.14; moderate-certainty evidence) probably result in a slight increase in the number of people who discontinue treatment due to adverse events. Serious adverse events (SAEs) were reported in 35 studies (33,998 participants). There was probably a trivial reduction in SAEs amongst people with RRMS treated with interferon beta-1b as compared to placebo (OR 0.92, 95% CI 0.55 to 1.54; moderate-certainty evidence).
Authors' conclusions: We are highly confident that, compared to placebo, two-year treatment with natalizumab, cladribine, or alemtuzumab decreases relapses more than with other DMTs. We are moderately confident that a two-year treatment with natalizumab may slow disability progression. Compared to those on placebo, people with RRMS treated with most of the assessed DMTs showed a higher frequency of treatment discontinuation due to AEs: we are moderately confident that this could happen with fingolimod, teriflunomide, interferon beta-1a, laquinimod, natalizumab and daclizumab, while our certainty with other DMTs is lower. We are also moderately certain that treatment with alemtuzumab is associated with fewer discontinuations due to adverse events than placebo, and moderately certain that interferon beta-1b probably results in a slight reduction in people who experience serious adverse events, but our certainty with regard to other DMTs is lower. Insufficient evidence is available to evaluate the efficacy and safety of DMTs in a longer term than two years, and this is a relevant issue for a chronic condition like MS that develops over decades. More than half of the included studies were sponsored by pharmaceutical companies and this may have influenced their results. Further studies should focus on direct comparison between active agents, with follow-up of at least three years, and assess other patient-relevant outcomes, such as quality of life and cognitive status, with particular focus on the impact of sex/gender on treatment effects.
Copyright © 2023 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
MGL: none known.
GF: no relevant interests; Joint Co‐ordinating Editor of Cochrane Multiple Sclerosis and Rare Disease of the CNS (not involved in the editorial process of this review); involved with MAIN 2014 ‐ Azathioprine versus beta interferons for relapsing‐remitting multiple sclerosis: a multicentre randomized non‐inferiority trial. PLoS One 2014;9(11):e113371. Funding: AIFA (Italian Medicines Agency)). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Trial Registration: EudraCT 2006‐004937‐13. This study was approved by ethics committees in the co‐ordinating centre (Careggi University Hospital, Ethics Committee, Florence) and each of the participating centres (Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano; Clinica Neurologica, Novara; Universita ‘‘La Sapienza’’, Roma; Policlinico ‘‘G. Rodolico’’ Azienda Ospedaliero‐Universitaria, Catania; Clinica Neurologica 2, Genova; Azienda Ospedaliera Universitaria Integrata, Verona; Ospedale Clinicizzato ‘‘Colle Dall’Ara’’, Chieti; Universita` di Sassari, Sassari; Universita` di Napoli, Napoli; Ospedale S. Antonio, Padova; Ospedale Civile S. Agostino‐Estense, Modena; Ospedale Santa Maria, Reggio Emilia; Policlinico Universitario Mater Domini, Catanzaro; Ospedale S. Gerardo, Monza; Azienda Ospedaliero‐Universitaria S. Anna, Ferrara; Ospedali Riuniti, Ancona; Istituto S. Raffaele ‘‘G. Giglio’’, Cefalu; Azienda Ospedaliero San Giovanni Battista, Universita` di Torino, Torino; Ospedale Sacro Cuore, Negrar; Ospedale Santa Chiara, Trento; Ospedale Regionale, Bolzano; Azienda Ospedaliero‐Universitaria Senese, Policlinico ‘‘Le Scotte’’, Siena; Ospedale ‘‘Misericordia e Dolce’’, Prato; Universita degli Studi di Pisa, Pisa; Policlinico ‘‘G. Martino’’, Messina; Universita degli Studi di Palermo, Palermo; Universita Cattolica, Policlinico Gemelli, Roma; Dipartimento Neuroriabilitativo ASL CN1, Cuneo; Luigi Gonzaga Hospital, Orbassano Ethics Committees), adhered to Good Clinical Practice (GCP) guidelines and Declaration of Helsinki.
CDG: Multiple Sclerosis International Federation (grant/contract).
BR: no relevant interests; Managing Editor of Cochrane Multiple Sclerosis and Rare Diseases of the CNS (not involved in the editorial process of this review).
FN: no relevant interests; epidemiologist and neurologist within the public Italian Health Service (at the IRCCS Istituto delle Scienze Neurologiche di Bologna); Co‐ordinating Editor of Cochrane Multiple Sclerosis and Rare Disease of the CNS (not involved in the editorial process of this review).
SM: no relevant interests; Joint Co‐ordinating Editor and Methods Editor of Cochrane Drugs and Alcohol.
GP: Bristol‐Myers Squibb (consultation); Multiple Sclerosis International Federation (patient representative and consultant); Multiple Sclerosis Society (patient representative and consultant); National Institute for Health Research (patient representative); published 'Celani MG, Nonino F, Mahan K, Orso M, Ridley B, Baldin E, et al. Identifying unanswered questions and setting the agenda for future systematic research in Multiple Sclerosis. A worldwide, multi‐stakeholder Priority Setting project. Mult Scler Relat Disord. 2022;60:103688'; 'Li V, Leurent B, Barkhof F, Braisher M, Cafferty F, Ciccarelli O, et al. Designing Multi‐arm Multistage Adaptive Trials for Neuroprotection in Progressive Multiple Sclerosis. Neurology. 2022;98(18):754‐764' and 'Alexander S, Peryer G, Gray E, Barkhof F, Chataway J. Wearable technologies to measure clinical outcomes in multiple sclerosis: A scoping review. Mult Scler. 2021;27(11):1643‐1656'; volunteered for the UK MS Society; acted as a peer reviewer for Cochrane.
TP: Multiple Sclerosis International Federation (grant/contract).
MF: Novartis, Merck, (travel); published an opinion paper on dalfampridine ‐ see Foschi M, Lugaresi A. Evaluating dalfampridine for the treatment of relapsing‐remitting multiple sclerosis: does it add to the treatment armamentarium? Expert Opinion on Pharmacotherapy, 2019 Jun; Consultant Neurologist at S. Maria delle Croci Hospital of Ravenna, AUSL Romagna, Ravenna, Italy.
EB: no relevant interests; author of a manuscript on ponesimod for the treatment of relapsing multiple sclerosis; Neurologist IRCCS Istituto delle Scienze Neurologiche di Bologna; Affiliated Researcher of Cochrane Multiple Sclerosis and Rare Disease of the CNS (not involved in the editorial process of this review).
IT: none known
Figures




















Update of
-
Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2015 Sep 18;2015(9):CD011381. doi: 10.1002/14651858.CD011381.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2024 Jan 4;1:CD011381. doi: 10.1002/14651858.CD011381.pub3. PMID: 26384035 Free PMC article. Updated. Review.
Similar articles
-
Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2015 Sep 18;2015(9):CD011381. doi: 10.1002/14651858.CD011381.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2024 Jan 4;1:CD011381. doi: 10.1002/14651858.CD011381.pub3. PMID: 26384035 Free PMC article. Updated. Review.
-
Immunomodulators and immunosuppressants for progressive multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2024 Sep 10;9(9):CD015443. doi: 10.1002/14651858.CD015443.pub2. Cochrane Database Syst Rev. 2024. PMID: 39254048 Free PMC article.
-
Adverse effects of immunotherapies for multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2023 Nov 30;11(11):CD012186. doi: 10.1002/14651858.CD012186.pub2. Cochrane Database Syst Rev. 2023. PMID: 38032059 Review.
-
Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2013 Jun 6;(6):CD008933. doi: 10.1002/14651858.CD008933.pub2. Cochrane Database Syst Rev. 2013. PMID: 23744561 Review.
-
Treatment with disease-modifying drugs for people with a first clinical attack suggestive of multiple sclerosis.Cochrane Database Syst Rev. 2017 Apr 25;4(4):CD012200. doi: 10.1002/14651858.CD012200.pub2. Cochrane Database Syst Rev. 2017. PMID: 28440858 Free PMC article. Review.
Cited by
-
Treatment effect modifiers of immunotherapies for relapsing-remitting multiple sclerosis-A systematic review and meta-analysis.Mult Scler J Exp Transl Clin. 2024 Oct 24;10(4):20552173241274618. doi: 10.1177/20552173241274618. eCollection 2024 Oct-Dec. Mult Scler J Exp Transl Clin. 2024. PMID: 39493424 Free PMC article. Review.
-
Risk of cancer development associated with disease-modifying therapies for multiple sclerosis: study protocol for a systematic review and meta-analysis of randomised and non-randomised studies.Syst Rev. 2024 Oct 18;13(1):263. doi: 10.1186/s13643-024-02677-z. Syst Rev. 2024. PMID: 39425150 Free PMC article.
-
Editorial: New drugs, approaches and strategies for multiple sclerosis treatment.Front Neurosci. 2024 Jan 30;18:1372140. doi: 10.3389/fnins.2024.1372140. eCollection 2024. Front Neurosci. 2024. PMID: 38352043 Free PMC article. No abstract available.
-
Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis.Cochrane Database Syst Rev. 2015 Sep 18;2015(9):CD011381. doi: 10.1002/14651858.CD011381.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2024 Jan 4;1:CD011381. doi: 10.1002/14651858.CD011381.pub3. PMID: 26384035 Free PMC article. Updated. Review.
References
References to studies included in this review
Achiron 1998 {published data only}
-
- Achiron A, Gabbay U, Gilad R, Hassin B, Barak Y, Gornish M, et al. Intravenous immunoglobulin treatment in multiple sclerosis. Effect on relapses. Neurology 1998;50(2):398-402. - PubMed
ADVANCE 2014 {published data only}
-
- Calabresi PA, Kieseier BC, Arnold DL, Balcer LJ, Boyko A, Pelletier J, et al. Pegylated interferon beta-1a for relapsing-remitting multiple sclerosis (ADVANCE): a randomised, phase 3, double-blind study. Lancet Neurology 2014;13:657-65. - PubMed
-
- Hu X, Cui Y, White J, Zhu Y, Deykin A, Nestorov I, Hung S. Pharmacokinetics and pharmacodynamics of peginterferon beta-1a in patients with relapsing-remitting multiple sclerosis in the randomized ADVANCE study. British Journal of Clinical Pharmacology 2015;79(3):514-22. [DOI: 10.1111/bcp.12521] [PMID: ] - DOI - PMC - PubMed
AFFIRM 2006 {published data only}
-
- Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. New England Journal of Medicine 2006;354(9):899-910. - PubMed
ALLEGRO 2012 {published data only}
-
- Comi G, Jeffery D, Kappos L, Montalban X, Boyko A, Rocca MA, et al. Placebo-controlled trial of oral laquinimod for multiple sclerosis. New England Journal of Medicine 2012;366(11):1000-9. - PubMed
-
- Filippi M, Rocca MA, Pagani E, De Stefano N, Jeffery D, Kappos L, et al, ALLEGRO Study Group. Placebo-controlled trial of oral laquinimod in multiple sclerosis: MRI evidence of an effect on brain tissue damage. J Neurol Neurosurg Psychiatry 2014;85(8):851-8. [DOI: 10.1136/jnnp-2013-306132] [PMID: ] - DOI - PubMed
ASCLEPIOS I 2020 {published data only}
ASCLEPIOS II 2020 {published data only}
ASSESS 2020 {published data only}
-
- Cree BAC, Goldman MD, Corboy JR, Singer BA, Fox EJ, Arnold DL, et al, ASSESS Trial Investigators. Efficacy and Safety of 2 Fingolimod Doses vs Glatiramer Acetate for the Treatment of Patients With Relapsing-Remitting Multiple Sclerosis: A Randomized Clinical Trial. JAMA Neurol 2020 ;78(1):1–13. [DOI: 10.1001/jamaneurol.2020.2950] [PMID: ] - DOI - PMC - PubMed
BECOME 2009 {published data only}
-
- Cadavid D, Wolansky LJ, Skurnick J, Lincoln J, Cheriyan J, Szczepanowski K, et al. Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology 2009;72(23):1976-83. - PubMed
BEYOND 2009 {published data only}
-
- O'Connor P, Filippi M, Arnason B, Comi G, Cook S, Goodin D, et al. 250 microg or 500 microg interferon beta-1b versus 20 mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurology 2009;8(10):889-97. - PubMed
Bornstein 1987 {published data only}
-
- Bornstein MB, Miller A, Slagle S, Weitzman M, Crystal, Drexler E, et al. A pilot trial of Cop 1 in exacerbating-remitting multiple sclerosis. New England Journal of Medicine 1987;317(7):408-14. - PubMed
BRAVO 2014 {published data only}
-
- Vollmer TL, Sorensen PS, Selmaj K, Zipp F, Havrdova E, Cohen JA, et al. A randomized placebo-controlled phase III trial of oral laquinimod for multiple sclerosis. Journal of Neurology 2014;261(4):773-83. - PubMed
CAMMS223 2008 {published data only}
-
- CAMMS223 Trial Investigators, Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. New England Journal of Medicine 2008;359(17):1786-801. - PubMed
CARE‐MS I 2012 {published data only}
-
- Arroyo González R, Kita M, Crayton H, Havrdova E, Margolin DH, Lake SL, et al, CARE-MS I and II Investigators. Alemtuzumab improves quality-of-life outcomes compared with subcutaneous interferon beta-1a in patients with active relapsing-remitting multiple sclerosis. Mult Scler 2017;23(10):1367-1376. [DOI: 10.1177/1352458516677589] - DOI - PubMed
-
- Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012;380(9856):1819-28. - PubMed
CARE‐MS II 2012 {published data only}
-
- Arroyo González R, Kita M, Crayton H, Havrdova E, Margolin DH, Lake SL, et al, CARE-MS I and II Investigators. Alemtuzumab improves quality-of-life outcomes compared with subcutaneous interferon beta-1a in patients with active relapsing-remitting multiple sclerosis. Mult Scler 2017;10:1367-1376. [DOI: 10.1177/1352458516677589] - DOI - PubMed
-
- Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012;380(9856):1829-39. - PubMed
CLARITY 2010 {published data only}
-
- De Stefano N, Giorgio A, Battaglini M, De Leucio A, Hicking C, Dangond F, et al. Reduced brain atrophy rates are associated with lower risk of disability progression in patients with relapsing multiple sclerosis treated with cladribine tablets. Mult Scler 2018;24(2):222-226. [DOI: 10.1177/1352458517690269] [PMID: ] - DOI - PMC - PubMed
-
- Giovannoni G, Soelberg Sorensen P, Cook S, Rammohan KW, Rieckmann P, Comi G, et al. Efficacy of Cladribine Tablets in high disease activity subgroups of patients with relapsing multiple sclerosis: A post hoc analysis of the CLARITY study. Mult Scler 2019;25(6):819-827. [DOI: 10.1177/1352458518771875] [PMID: ] - DOI - PMC - PubMed
CombiRx 2013 {published data only}
CONCERTO 2021 {published data only}
-
- NCT01707992. The Efficacy, Safety, and Tolerability of Laquinimod in Subjects With Relapsing Remitting Multiple Sclerosis (RRMS). https://clinicaltrials.gov/show/NCT01707992 2012.
CONFIRM 2012 {published data only}
-
- Fernández Ó, Giovannoni G, Fox RJ, Gold R, Phillips JT, Potts J, et al. Efficacy and Safety of Delayed-release Dimethyl Fumarate for Relapsing-remitting Multiple Sclerosis in Prior Interferon Users: An Integrated Analysis of DEFINE and CONFIRM. Clin Ther 2017;39(8):1671-1679. [DOI: 10.1016/j.clinthera.2017.06.012] - DOI - PubMed
-
- Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. New England Journal of Medicine 2012;367(12):1087-97. - PubMed
-
- Havrdova E, Giovannoni G, Gold R, Fox RJ, Kappos L, Phillips JT, et al. Effect of delayed-release dimethyl fumarate on no evidence of disease activity in relapsing-remitting multiple sclerosis: integrated analysis of the phase III DEFINE and CONFIRM studies. Eur J Neurol 2017;24(5):726-733. [DOI: 10.1111/ene.13272] - DOI - PMC - PubMed
-
- Hutchinson M, Fox RJ, Miller DH, Phillips JT, Kita M, Havrdova E, et al. Clinical efficacy of BG-12 (dimethyl fumarate) in patients with relapsing-remitting multiple sclerosis: subgroup analyses of the CONFIRM study. J Neurol 2013;260(9):2286-96. [DOI: 10.1007/s00415-013-6968-1] [PMID: ] - DOI - PubMed
-
- Kita M, Fox RJ, Phillips JT, Hutchinson M, Havrdova E, Sarda SP, et al. Effects of BG-12 (dimethyl fumarate) on health-related quality of life in patients with relapsing-remitting multiple sclerosis: findings from the CONFIRM study. Mult Scler 2014;20(2):253-7. [DOI: 10.1177/1352458513507818] [PMID: ] - DOI - PubMed
DECIDE 2015 {published data only}
-
- Kappos L, Havrdova E, Giovannoni G, Khatri BO, Gauthier SA, Greenberg SJ, et al. No evidence of disease activity in patients receiving daclizumab versus intramuscular interferon beta-1a for relapsing-remitting multiple sclerosis in the DECIDE study. Mult Scler 2017;23(13):1736-1747. [DOI: 10.1177/1352458516683266] - DOI - PubMed
-
- Krueger JG, Kircik L, Hougeir F, Friedman A, You X, Lucas N, et al. Cutaneous Adverse Events in the Randomized, Double-Blind, Active-Comparator DECIDE Study of Daclizumab High-Yield Process Versus Intramuscular Interferon Beta-1a in Relapsing-Remitting Multiple Sclerosis. Adv Ther 2016;33(7):231-45. [DOI: 10.1007/s12325-016-0353-2] - DOI - PMC - PubMed
DEFINE 2012 {published data only}
-
- Bar-Or A, Gold R, Kappos L, Arnold DL, Giovannoni G, Selmaj K, et al. Clinical efficacy of BG-12 (dimethyl fumarate) in patients with relapsing-remitting multiple sclerosis: subgroup analyses of the DEFINE study. J Neurol 2013 ;260(9):2297-305. [DOI: 10.1007/s00415-013-6954-7] [PMID: ] - DOI - PubMed
-
- Fernández Ó, Giovannoni G, Fox RJ, Gold R, Phillips JT, Potts J, et al. Efficacy and Safety of Delayed-release Dimethyl Fumarate for Relapsing-remitting Multiple Sclerosis in Prior Interferon Users: An Integrated Analysis of DEFINE and CONFIRM. Clin Ther 2017;39(8):1671-1679. [DOI: 10.1016/j.clinthera.2017.06.012] [PMID: ] - DOI - PubMed
-
- Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. New England Journal of Medicine 2012;367(12):1098-107. - PubMed
Etemadifar 2006 {published data only}
-
- Etemadifar M, Janghorbani M, Shaygannejad V. Comparison of Betaferon, Avonex, and Rebif in treatment of relapsing-remitting multiple sclerosis. Acta Neurologica Scandinavica 2006;113(5):283-7. - PubMed
Etemadifar 2007 {published data only}
-
- Etemadifar M, Janghorbani M, Shaygannejad V. Comparison of interferon beta products and azathioprine in the treatment of relapsing-remitting multiple sclerosis. Journal of Neurology 2007;254(12):1723-8. - PubMed
Fazekas 1997 {published data only}
-
- Fazekas F, Deisenhammer F, Strasser-Fuchs S, Nahler G, Mamoli B. Randomised placebo-controlled trial of monthly intravenous immunoglobulin therapy in relapsing-remitting multiple sclerosis. Lancet 1997;349(9052):589-93. - PubMed
FREEDOMS 2010 {published data only}
-
- Devonshire V, Havrdova E, Radue EW, O'Connor P, Zhang-Auberson L, Agoropoulou C, et al, FREEDOMS study group. Relapse and disability outcomes in patients with multiple sclerosis treated with fingolimod: subgroup analyses of the double-blind, randomised, placebo-controlled FREEDOMS study. Lancet Neurol 2012;11(5):420-8. [DOI: 10.1016/S1474-4422(12)70056-X] [PMID: ] - DOI - PubMed
-
- Kappos L, Radue EW, O'Connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. New England Journal of Medicine 2010;362(5):387-401. - PubMed
-
- Kremenchutzky M, O'Connor P, Hohlfeld R, Zhang-Auberson L, Rosenstiel P, Meng X, et al. Impact of prior treatment status and reasons for discontinuation on the efficacy and safety of fingolimod: Subgroup analyses of the Fingolimod Research Evaluating Effects of Daily Oral Therapy in Multiple Sclerosis (FREEDOMS) study. Mult Scler Relat Disord 2014;3(3):341-9. [DOI: 10.1016/j.msard.2013.10.006] [PMID: ] - DOI - PubMed
-
- Radue EW, O'Connor P, Polman CH, Hohlfeld R, Calabresi P, Selmaj K, Mueller-Lenke N, Agoropoulou C, Holdbrook F, Vera A, Zhang-Auberson L, Francis G, Burtin P, Kappos L, FTY720 Research Evaluating Effects of Daily Oral Therapy in Multiple Sclerosis (FREEDOMS) Study Group. Impact of fingolimod therapy on magnetic resonance imaging outcomes in patients with multiple sclerosis. Arch Neurol 2012;69(10):1259-69. [PMID: 10.1001/archneurol.2012.1051] [PMID: ] - DOI - PubMed
FREEDOMS II 2014 {published data only}
-
- Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurology 2014;13(6):545-56. - PubMed
GALA 2013 {published data only}
-
- Khan O, Rieckmann P, Boyko A, Selmaj K, Zivadinov R, GALA Study Group. Three times weekly glatiramer acetate in relapsing-remitting multiple sclerosis. Annals of Neurology 2013;73(6):705-13. - PubMed
-
- Zivadinov R, Dwyer M, Barkay H, Steinerman JR, Knappertz V, Khan O. Effect of glatiramer acetate three-times weekly on the evolution of new, active multiple sclerosis lesions into T1-hypointense "black holes": a post hoc magnetic resonance imaging analysis. J Neurol 2015;262(3):648-53. [DOI: 10.1007/s00415-014-7616-0] [PMID: ] - DOI - PubMed
Gobbi 2013 {published data only}
GOLDEN 2017 {published data only}
-
- Comi G, Patti F, Rocca MA, Mattioli FC, Amato MP, Gallo P, Centonze D, Pozzilli C, Saccà F, Bergh FT, Bartezaghi M, Turrini R, Filippi M, Golden Study Group. Efficacy of fingolimod and interferon beta-1b on cognitive, MRI, and clinical outcomes in relapsing-remitting multiple sclerosis: an 18-month, open-label, rater-blinded, randomised, multicentre study (the GOLDEN study). J Neurol 2017;264(12):2436-2449. [DOI: 10.1007/s00415-017-8642-5] [PMID: ] - DOI - PMC - PubMed
Goodkin 1991 {published data only}
-
- Goodkin D, Bailly R, Teetzen M, Hertsgaard D, Beatty W. The efficacy of azathioprine in relapsing-remitting multiple sclerosis. Neurology 1991;41:20-5. - PubMed
IFNB MS Group 1993 {published data only}
-
- IFNB MSG. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFNB Multiple Sclerosis Study Group. Neurology 1993;43(4):655-61. - PubMed
INCOMIN 2002 {published data only}
-
- Durelli L, Verdun E, Barbero P, Bergui M, Versino E, Ghezzi A, et al. Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN). Lancet 2002;359:1453-60. - PubMed
Johnson 1995 {published data only}
-
- Johnson K, Brooks B, Cohen J, Ford C, Goldstein J, Lisak R, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology 1995;45(7):1268-76. - PubMed
Knobler 1993 {published data only}
-
- Knobler RL, Greenstein JI, Johnson KP, Lublin FD, Panitch HS, Conway K, et al. Systemic recombinant human interferon-beta treatment of relapsing-remitting multiple sclerosis: pilot study analysis and six-year follow-up. Journal of Interferon Research 1993;13(5):333-40. - PubMed
Koch‐Henriksen 2006 {published data only}
-
- Koch-Henriksen N, Sørensen P, Christensen T, Frederiksen J, Ravnborg M, Jensen K, et al. A randomised study of two interferon-beta treatments in relapsing-remitting multiple sclerosis. Neurology 2006;66(7):1056-60. - PubMed
Lewanska 2002 {published data only}
-
- Lewanska M, Siger-Zajdel M, Selmaj K. No difference in efficacy of two different doses of intravenous immunoglobulins in MS: clinical and MRI assessment. European Journal of Neurology 2002;9(6):565-72. - PubMed
MAIN 2014 {published data only}
Millefiorini 1997 {published data only}
-
- Millefiorini E, Gasperini C, Pozzilli C, D'Andrea F, Bastianello S, Trojano M, et al. Randomized placebo-controlled trial of mitoxantrone in relapsing-remitting multiple sclerosis: 24-month clinical and MRI outcome. Journal of Neurology 1997;244(3):153-9. - PubMed
Mokhber 2014 {published data only}
-
- Mokhber N, Azarpazhooh A, Orouji E, Rao SM, Khorram B, Sahraian MA, Foroghipoor M, Gharavi MM, Kakhi S, Nikkhah K, Azarpazhooh MR. Cognitive dysfunction in patients with multiple sclerosis treated with different types of interferon beta: a randomized clinical trial. J Neurol Sci 2014;342(1-2):16-20. [DOI: 10.1016/j.jns.2014.01.038] [PMID: ] - DOI - PubMed
MSCRG 1996 {published data only}
-
- Jacobs L, Cookfair D, Rudick R, Herndon R, Richert J, Salazar A, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Annals of Neurology 1996;39:285-94. - PubMed
OPERA I 2017 {published data only}
-
- A randomized, double-blind, double-dummy, parallel-group study to evaluate the efficacy and safety of Ocrelizumab in comparison to interferon beta-1a (Rebif®) in patients with relapsing multiple sclerosis. https://clinicaltrials.gov/ct2/show/study/NCT01247324 (accessed 30 September 2014).
-
- Mayer L, Kappos L, Racke MK, Rammohan K, Traboulsee A, Hauser SL, Julian L, Köndgen H, Li C, Napieralski J, Zheng H, Wolinsky JS. Ocrelizumab infusion experience in patients with relapsing and primary progressive multiple sclerosis: Results from the phase 3 randomized OPERA I, OPERA II, and ORATORIO studies.. Mult Scler Relat Disord 2019;30:236-243. [DOI: 10.1016/j.msard.2019.01.044] [PMID: ] - DOI - PubMed
OPERA II 2017 {published data only}
-
- Mayer L, Kappos L, Racke MK, Rammohan K, Traboulsee A, Hauser SL, et al. Ocrelizumab infusion experience in patients with relapsing and primary progressive multiple sclerosis: Results from the phase 3 randomized OPERA I, OPERA II, and ORATORIO studies. Mult Scler Relat Disord 2019;30:236-243. [DOI: 10.1016/j.msard.2019.01.044] [PMID: ] - DOI - PubMed
-
- NCT01412333. A randomized, double-blind, double-dummy, parallel-group study to evaluate the efficacy and safety of Ocrelizumab in comparison to Interferon Beta-1a (Rebif®) in patients with relapsing multiple sclerosis. https://clinicaltrials.gov/ct2/show/study/NCT01412333 (accessed 30 September 2014).
OPTIMUM 2021 {published data only}
-
- Kappos L, Fox RJ, Burcklen M, Freedman MS, Havrdová EK, Hennessy B, et al. Ponesimod Compared With Teriflunomide in Patients With Relapsing Multiple Sclerosis in the Active-Comparator Phase 3 OPTIMUM Study: A Randomized Clinical Trial. JAMA Neurol 2021 May 1;;78(5):558-567. [DOI: 10.1001/jamaneurol.2021.0405] [PMID: ] - DOI - PMC - PubMed
PRISMS 1998 {published data only}
-
- Traboulsee A, Li DKB, Cascione M, Fang J, Dangond F, Miller A. Effect of interferon beta-1a subcutaneously three times weekly on clinical and radiological measures and no evidence of disease activity status in patients with relapsing-remitting multiple sclerosis at year 1. BMC Neurol 2018;18(1):143. [DOI: 10.1186/s12883-018-1145-x] [PMID: ] - DOI - PMC - PubMed
RADIANCE 2019 {published data only}
-
- Cohen JA, Comi G, Selmaj KW, Bar-Or A, Arnold DL, Steinman L, et al, RADIANCE Trial Investigators. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol 2019;18(11):1021-1033. [DOI: 10.1016/S1474-4422(19)30238-8] [PMID: ] - DOI - PubMed
-
- NCT01628393. Efficacy and Safety Study of RPC1063 in Relapsing Multiple Sclerosis Patients (Radiance Study). https://clinicaltrials.gov/show/NCT01628393 2012.
REGARD 2008 {published data only}
-
- Mikol D, Barkhof F, Chang P, Coyle P, Jeffery D, Schwid S, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurology 2008;7(10):903-14. - PubMed
SELECT 2013 {published data only}
-
- Giovannoni G, Kappos L, Gold R, Khatri BO, Selmaj K, Umans K, et al. Safety and tolerability profile of daclizumab in patients with relapsing-remitting multiple sclerosis: An integrated analysis of clinical studies. Mult Scler Relat Disord 2016 ;9:36-46. [DOI: 10.1016/j.msard.2016.05.010] - DOI - PubMed
-
- Gold R, Giovannoni G, Selmaj K, Havrdova E, Montalban X, Radue EW, et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial. Lancet 2013;381(9884):2167-75. - PubMed
-
- Phillips G, Guo S, Bender R, Havrdová E, Proskorovsky I, Vollmer T. Assessing the impact of multiple sclerosis disease activity and daclizumab HYP treatment on patient-reported outcomes: Results from the SELECT trial. Mult Scler Relat Disord 2016 ;6:66-72. [DOI: 10.1016/j.msard.2016.02.001] - DOI - PubMed
SUNBEAM 2019 {published data only}
-
- Comi G, Kappos L, Selmaj KW, Bar-Or A, Arnold DL, Steinman L, et al, SUNBEAM Study Investigators. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurology 2019 ;18(11):1009-1020. [DOI: 10.1016/S1474-4422(19)30239-X] - DOI - PubMed
-
- NCT02294058. Study of Ozanimod (RPC1063) in Relapsing Multiple Sclerosis (MS). https://ClinicalTrials.gov/show/NCT02294058 2014.
TEMSO 2011 {published data only}
-
- Miller AE, O'Connor P, Wolinsky JS, Confavreux C, Kappos L, Olsson TP, et al, Teriflunomide Multiple Sclerosis Trial Group. Pre-specified subgroup analyses of a placebo-controlled phase III trial (TEMSO) of oral teriflunomide in relapsing multiple sclerosis. Mult Scler 2012;18(11):1625-32. [DOI: 10.1177/1352458512450354] [PMID: ] - DOI - PMC - PubMed
-
- O'Connor P, Wolinsky JS, Confavreux C, Comi G, Kappos L, Olsson TP, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. New England Journal of Medicine 2011;365(14):1293-303. - PubMed
-
- Wolinsky JS, Narayana PA, Nelson F, Datta S, O'Connor P, Confavreux C, et al, Teriflunomide Multiple Sclerosis Oral (TEMSO) Trial Group. Magnetic resonance imaging outcomes from a phase III trial of teriflunomide. Mult Scler 2013 ;19(10):1310-9. [DOI: 10.1177/1352458513475723] [PMID: ] - DOI - PubMed
TOWER 2014 {published data only}
-
- Confavreux C, O'Connor P, Comi G, Freedman MS, Miller AE, Olsson TP, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurology 2014;13(3):247-56. - PubMed
-
- Miller AE, Xu X, Macdonell R, Vucic S, Truffinet P, Benamor M, et al. Efficacy and safety of teriflunomide in Asian patients with relapsing forms of multiple sclerosis: A subgroup analysis of the phase 3 TOWER study. J Clin Neurosci 2019;59:229-231. [DOI: 10.1016/j.jocn.2018.09.012] [PMID: ] - DOI - PubMed
-
- Qiu W, Huang DH, Hou SF, Zhang MN, Jin T, Dong HQ, et al, TOWER Trial Chinese Group. Efficacy and Safety of Teriflunomide in Chinese Patients with Relapsing Forms of Multiple Sclerosis: A Subgroup Analysis of the Phase 3 TOWER Study. Chin Med J (Engl) 2018;131(23):2776-2784. [DOI: 10.4103/0366-6999.246067] [PMID: ] - DOI - PMC - PubMed
TRANSFORMS 2010 {published data only}
-
- Barkhof F, Jong R, Sfikas N, Vera A, Francis G, Cohen J, TRANSFORMS study group. The influence of patient demographics, disease characteristics and treatment on brain volume loss in Trial Assessing Injectable Interferon vs FTY720 Oral in Relapsing-Remitting Multiple Sclerosis (TRANSFORMS), a phase 3 study of fingolimod in multiple sclerosis. Mult Scler 2014;20(13):1704-13. [DOI: 10.1177/1352458514532317] [PMID: ] - DOI - PubMed
-
- Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. New England Journal of Medicine 2010;362(5):402-15. - PubMed
-
- Khatri BO, Pelletier J, Kappos L, Hartung HP, Comi G, Barkhof F, et al, TRANSFORMS Study Group. Effect of prior treatment status and reasons for discontinuation on the efficacy and safety of fingolimod vs. interferon β-1a intramuscular: Subgroup analyses of the Trial Assessing Injectable Interferon vs. Fingolimod Oral in Relapsing-Remitting Multiple Sclerosis (TRANSFORMS). Mult Scler Relat Disord 2014;3(3):355-63. [DOI: 10.1016/j.msard.2013.11.006] [PMID: ] - DOI - PubMed
-
- Meng X, Chin PS, Hashmonay R, Zahur Islam M, Cutter G. Effect of switching from intramuscular interferon β-1a to oral fingolimod on time to relapse in patients with relapsing-remitting multiple sclerosis enrolled in a 1-year extension of TRANSFORMS. Contemp Clin Trials 2015;41:69-74. [DOI: 10.1016/j.cct.2014.12.011] [PMID: ] - DOI - PubMed
References to studies excluded from this review
ACT 2009 {published data only}
-
- Cohen JA, Imrey PB, Calabresi PA, Edwards KR, Eickenhorst T, Felton WL 3rd, et al. Results of the Avonex Combination Trial (ACT) in relapsing-remitting MS. Neurology 2009;72(6):535-41. - PubMed
Agius 2014 {published data only}
Ashtari 2011 {published data only}
ATAMS 2014 {published data only}
-
- Kappos L, Hartung HP, Freedman MS, Boyko A, Radü EW, Mikol DD, et al. Atacicept in multiple sclerosis (ATAMS): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Neurology 2014;13(4):353-63. - PubMed
Bar‐Or 2017 {published data only}
-
- Bar-Or A, Grove RA, Austin DJ, Tolson JM, VanMeter SA, Lewis EW, Derosier FJ, Lopez MC, Kavanagh ST, Miller AE, Sorensen PS. Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: The MIRROR study.. Neurology. 2018 ;90(20):e1805-e1814. [DOI: 10.1212/WNL.0000000000005516] - DOI - PMC - PubMed
Boiko 2018 {published data only}
-
- Boiko AN, Bosenko LP, Vasilovskii VV, Volkova LI, Zakharova MN, Kotov SV, Lekomtseva EV, Negrich TI, Parshina EV, Patrusheva OP, Prokopenko SV, Sazonov DV, Timchenko PV, Trinitatskii YV, Khabirov FA, Khavunka MY, Chichanovskaya LV, Sherman MA, Lin'kova YN, Zinkina‐Orikhan AV, Tursunova KB. A Comparative Placebo-Controlled Clinical Trial of the Efficacy and Safety of Interferon β-1a Formulations for S.C. Administration in Patients with Remitting Multiple Sclerosis: first-Year Results. Neuroscience and behavioral physiology 2018;48(7):883‐889.
Boyko 2016 {published data only}
-
- Boyko AN, Lashch NY, Sharanova SN, Zakharova MN, Trifonova OV, Simaniv TO, Lysogorskaya EV, Guryanova OE, Kotov SV, Iakushina TI, Lizhdvoy VY, Belova YA, Khabirov FA, Babicheva NN, Khaibullin TI, Granatov EV, Averyanova LA, Sazonov DV, Odinak MM, Trinitatsky YV, Tsukurova LA, Sergeeva AI, Ivanov RA, Shustova MS. [Comparative, placebo-controlled clinical study of efficacy and safety of glatiramer acetate 20 mg in patients with relapsing-remitting multiple sclerosis: results of the first year of the study] [Sravnitel'noe platsebo-kontroliruemoe klinicheskoe issledovanie effektivnosti i bezopasnosti preparatov glatiramera atsetata 20 mg u patsientov s remittiruyushchim rasseyannym sklerozom: rezul'taty pervogo goda nablyudeniya]. Zh Nevrol Psikhiatr Im S S Korsakova. 2016;116(10 Pt 2):61-67. [DOI: 10.17116/jnevro201611610261-67] - DOI - PubMed
British and Dutch 1988 {published data only}
-
- The British, Dutch MSATG. Double-masked trial of azathioprine in multiple sclerosis. Lancet 1988;Vol. 2(8604):179–83. - PubMed
Calabrese 2012 {published data only}
-
- Calabrese M, Bernardi V, Atzori M, Mattisi I, Favaretto A, Rinaldi F, et al. Effect of disease-modifying drugs on cortical lesions and atrophy in relapsing-remitting multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England) 2012;18(4):418-24. - PubMed
Cascione 2018 {published data only}
-
- Cascione M, Tenenbaum N, Wendt J, Meng X, Schofield L, Cree BAC, PREFERMS investigators. Treatment retention on fingolimod compared with injectable multiple sclerosis therapies in African-American patients: A subgroup analysis of a randomized phase 4 study.. Mult Scler Relat Disord. 2018 ;25:50-56. [DOI: 10.1016/j.msard.2018.07.014] - DOI - PubMed
CHOICE 2010 {published data only}
-
- Wynn D, Kaufman M, Montalban X, Vollmer T, Simon J, Elkins J, et al. Daclizumab in active relapsing multiple sclerosis (CHOICE study): a phase 2, randomised, double-blind, placebo-controlled, add-on trial with interferon beta. Lancet Neurology 2010;9(4):381-90. - PubMed
Cohen 2015 {published data only}
-
- Cohen J, Belova A, Selmaj K, Wolf C, Sormani MP, Oberyé J, den Tweel E, Mulder R, Koper N, Voortman G, Barkhof F, Glatiramer Acetate Clinical Trial to Assess Equivalence With Copaxone (GATE) Study Group. Equivalence of Generic Glatiramer Acetate in Multiple Sclerosis: A Randomized Clinical Trial.. JAMA Neurol 2015;72(12):1433-41. [DOI: 10.1001/jamaneurol.2015.2154] - DOI - PubMed
Cohen 2016 {published data only}
-
- Cohen JA, Arnold DL, Comi G, Bar-Or A, Gujrathi S, Hartung JP, Cravets M, Olson A, Frohna PA, Selmaj KW, RADIANCE Study Group. Safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ozanimod in relapsing multiple sclerosis (RADIANCE): a randomised, placebo-controlled, phase 2 trial.. Lancet Neurol. 2016 ;15(4):373-81. [DOI: 10.1016/S1474-4422(16)00018-1] - DOI - PubMed
Comi 2001 {published data only}
-
- Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging--measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer Acetate Study Group. Annals of Neurology 2001;49(3):290-7. [PMID: ] - PubMed
Coyle 2017 {published data only}
-
- Patricia K Coyle, Anthony T Reder, Mark S Freedman, Juanzhi Fang, Fernando Dangond. Early MRI results and odds of attaining ‘no evidence of disease activity’ status in MS patients treated with interferon β-1a in the EVIDENCE study. Journal of the Neurological Sciences 2017;379:151-156. [DOI: 10.1016/j.jns.2017.05.052] - DOI - PubMed
Cree 2018 {published data only}
-
- Cree BAC, Arnold DL, Cascione M, Fox EJ, Williams IM, Meng X, Schofield L, Tenenbaum N. Phase IV study of retention on fingolimod versus injectable multiple sclerosis therapies: a randomized clinical trial.. Ther Adv Neurol Disord. 2018;11:756286418774338. [DOI: 10.1177/1756286418774338] - DOI - PMC - PubMed
EVIDENCE 2007 {published data only}
-
- Schwid S, Panitch H. Full results of the Evidence of Interferon Dose-Response-European North American Comparative Efficacy (EVIDENCE) study: a multicenter, randomized, assessor-blinded comparison of low-dose weekly versus high-dose, high-frequency interferon beta-1a for relapsing multiple sclerosis. Clinical Therapeutics 2007;29(9):2031-48. - PubMed
Fazekas 2008 {published data only}
-
- Fazekas F, Lublin F, Li D, Freedman M, Hartung H, Rieckmann P, et al. Intravenous immunoglobulin in relapsing-remitting multiple sclerosis: a dose-finding trial. Neurology 2008;71(4):265-71. - PubMed
FORTE 2011 {published data only}
-
- Comi G, Cohen JA, Arnold DL, Wynn D, Filippi M, FORTE Study Group. Phase III dose-comparison study of glatiramer acetate for multiple sclerosis. Annals of Neurology 2011;69(1):75-82. - PubMed
Fox 2014 {published data only}
-
- Fox E, Edwards K, Burch G, Wynn DR, LaGanke C, Crayton H, Hunter SF, Huffman C, Kim E, Pestreich L, McCague K, Barbato L, EPOC study investigators. Outcomes of switching directly to oral fingolimod from injectable therapies: results of the randomized, open-label, multicenter, Evaluate Patient OutComes (EPOC) study in relapsing multiple sclerosis. Multiple sclerosis and related disorders 2014;3(5):607‐619. - PubMed
Freedman 2012 {published data only}
-
- Freedman MS, Wolinsky JS, Wamil B, Confavreux C, Comi G, Kappos L, et al. Teriflunomide added to interferon-β in relapsing multiple sclerosis: a randomized phase II trial. Neurology 2012;78(23):1877-85. - PubMed
Ghezzi 1989 {published data only}
-
- Ghezzi A, Di Falco M, Locatelli C In: Consette RE, Delmotte P editor(s). Clinical controlled randomized trial of azathioprine in multiple sclerosis. Elsevier 1989.
Havrdova 2009 {published data only}
-
- Havrdova E, Zivadinov R, Krasensky J, Dwyer MG, Novakova I, Dolezal O, et al. Randomized study of interferon beta-1a, low-dose azathioprine, and low-dose corticosteroids in multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England) 2009;15(8):965-76. - PubMed
IMPROVE 2010 {published data only}
-
- De Stefano N, Sormani MP, Stubinski B, Blevins G, Drulovic JS, Issard D, Shotekov P, Gasperini C. Efficacy and safety of subcutaneous interferon β-1a in relapsing-remitting multiple sclerosis: further outcomes from the IMPROVE study.. J Neurol Sci. 2012;312((1-2)):97-101. [DOI: 10.1016/j.jns.2011.08.013] [PMID: ] - DOI - PubMed
Kappos 2006 {published data only}
-
- Kappos L, Antel J, Comi G, Montalban X, O'Connor P, Polman CH, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. New Egyptian Journal of Medicine 2006;355(11):1124-40. - PubMed
Kappos 2008 {published data only}
-
- Kappos L, Gold R, Miller DH, Macmanus DG, Havrdova E, Limmroth V, et al. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 2008;372(9648):1463-72. - PubMed
Kappos 2011 {published data only}
-
- Kappos L, Li D, Calabresi PA, O'Connor P, Bar-Or A, Barkhof F, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 2011;378(9805):1779-87. - PubMed
Khoury 2010 {published data only}
Lampl 2013 {published data only}
-
- Lampl C, Nagl S, Arnason B, Comi G, O Connor P, Cook S, Jeffery D, Kappos L, Filippi M, Beckmann K, Bogumil T, Pohl C, Sandbrink R, Hartung HP. Efficacy and safety of interferon beta-1b sc in older RRMS patients--a posthoc analysis of the BEYOND study. J Neurol 2013;260(7):1838-45. [DOI: 10.1007/s00415-013-6888-0] - DOI - PubMed
Le Page 2015 {published data only}
-
- Le Page E, Veillard D, Laplaud DA, Hamonic S, Wardi R, Lebrun C, Zagnoli F, Wiertlewski S, Deburghgraeve V, Coustans M, Edan G, COPOUSEP investigators, West Network for Excellence in Neuroscience. Oral versus intravenous high-dose methylprednisolone for treatment of relapses in patients with multiple sclerosis (COPOUSEP): a randomised, controlled, double-blind, non-inferiority trial.. Lancet. 2015;386(9997):974-81. [DOI: 10.1016/S0140-6736(15)61137-0] - DOI - PubMed
Milanese 1993 {published data only}
-
- Milanese C, La Mantia L, Salmaggi A, Eoli M. A double blind study on azathioprine efficacy in multiple sclerosis: final report.. Journal of Neurology 1993;240(5):295–8. - PubMed
Newsome 2015 {published data only}
Ochi 2018 {published data only}
-
- Ochi H, Niino M, Onizuka Y, Hiramatsu K, Hase M, Yun J, Matta A, Torii S. 72-Week Safety and Tolerability of Dimethyl Fumarate in Japanese Patients with Relapsing-remitting Multiple Sclerosis: Analysis of the Randomised, Double Blind, Placebo-Controlled, Phase III APEX Study and its Open-Label Extension.. Adv Ther. 2018 ;35(10):1598-1611. [DOI: 10.1007/s12325-018-0788-8] - DOI - PMC - PubMed
OWIMS 1999 {published data only}
-
- OWIMS. Evidence of interferon beta-1a dose response in relapsing-remitting MS: the OWIMS Study. The Once Weekly Interferon for MS Study Group. Neurology 1999;53(4):679-86. - PubMed
Saida 2012 {published data only}
-
- Saida T, Kikuchi S, Itoyama Y, Hao Q, Kurosawa T, Nagato K, et al. A randomized, controlled trial of fingolimod (FTY720) in Japanese patients with multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England) 2012;18(9):1269-77. - PubMed
Saida 2017 {published data only}
-
- Saida T, Kira JI, Kishida S, Yamamura T, Ohtsuka N, Dong Q, Tibung JT. Natalizumab for Achieving Relapse-Free, T1 Gadolinium-Enhancing-Lesion-Free, and T2 Lesion-Free Status in Japanese Multiple Sclerosis Patients: A Phase 2 Trial Subanalysis.. Neurol Ther. 2017;6(1):153-159. [DOI: 10.1007/s40120-016-0062-4] - DOI - PMC - PubMed
SENTINEL 2006 {published data only}
-
- Rudick RA, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Radue EW, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. New England Journal of Medicine 2006;354(9):911-23. - PubMed
Simaniv 2019 {published data only}
-
- Simaniv, T O, Zakharova, M N, Boyko, A N, Lashch, NYu, Kotov, S V, Khabirov, F A, Khaibullin, T I, Sazonov, D V, Yarmoschuk, A V, Babenko, L A, et al. SAFETY ASPECTS WITHOUT LOSS OF EFFECTIVENESS IN THE SWITCH OF PATIENTS WITH MULTIPLE SCLEROSIS FROM THE ORIGINAL DRUG GLATIRAMER ACETATE COPAXONE-TEVA ON THE BIOSIMILAR TIMEXON. Russian neurological Journal 2019;0(4):44-51. [DOI: ]
Sorensen 2014 {published data only}
-
- Sorensen PS, Lisby S, Grove R, Derosier F, Shackelford S, Havrdova E, et al. Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase 2 study. Neurology 2014;82(7):573-81. - PubMed
TENERE 2014 {published data only}
-
- Vermersch P, Czlonkowska A, Grimaldi LM, Confavreux C, Comi G, Kappos L, et al. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Multiple Sclerosis (Houndmills, Basingstoke, England) 2014;20(6):705-16. - PubMed
Ziemssen 2017 {published data only}
-
- Ziemssen T, Tumani H, Sehr T, Thomas K, Paul F, Richter N, Samara E, Spiegelstein O, Sorani E, Bar-Ilan O, Mimrod D, Hayardeny L. Safety and in vivo immune assessment of escalating doses of oral laquinimod in patients with RRMS. J Neuroinflammation. 2017 ;31(14(1)):172. [DOI: 10.1186/s12974-017-0945-z] - DOI - PMC - PubMed
References to studies awaiting assessment
ACTRN12621001502820 {published data only}
-
- Reducing the frequency of Autoimmune adverse events in the treatment of Multiple sclerosis with alemtuzumab using B-celL dEpletion (RAMBLE): a phase II, randomised, placebo-controlled clinical trial. https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12621001502820.
Boyko 2022 {published data only}
-
- Boyko AN, Boyko OV, Bakhtiyarova KZ, Gusev EI, Dudin VA, Zaslavsky LG, Malkova NA, Parshina YV, Poverennova IY, Siverceva SA, Totolyan NA, Shchur SG, Fedulov AS, Khabirov FA, Bolsun DD, Zinkina-Orikhan AV, Linkova YN, Chernovskaya TV. Efficacy and safety of sampeginterferon β-1a in the treatment of relapsing remitting multiple sclerosis: results of 52 weeks of therapy in a randomized, double-blind clinical trial [Effektivnost' i bezopasnost' sampeginterferona β-1a dlya lecheniya remittiruyushchego rasseyannogo skleroza: rezul'taty 52-nedel'nogo randomizirovannogo dvoinogo slepogo klinicheskogo issledovaniya]. Zh Nevrol Psikhiatr Im S S Korsakova 2022;122(1):62-71. [DOI: 10.17116/jnevro202212201162] [PMID: ] - DOI - PubMed
CLARITY {published data only}
-
- De Stefano N, Sormani MP, Giovannoni G, Rammohan K, Leist T, Coyle PK, Dangond F, Keller B, Alexandri N, Galazka A. Analysis of frequency and severity of relapses in multiple sclerosis patients treated with cladribine tablets or placebo: The CLARITY and CLARITY Extension studies. Mult Scler 2022;28(1):111-120. [DOI: 10.1177/13524585211010294] [PMID: ] - DOI - PMC - PubMed
-
- Vermersch P, Galazka A, Dangond F, Damian D, Wong SL, Jack D, Harty G. Efficacy of cladribine tablets in high disease activity patients with relapsing multiple sclerosis: post hoc analysis of subgroups with and without prior disease-modifying drug treatment. Curr Med Res Opin 2021 ;37(3):459-464. [DOI: 10.1080/03007995.2020.1865888] [PMID: ] - DOI - PubMed
CombiRx {published data only}
EUCTR2017‐000559‐26‐IT {published data only}
-
- A multicentric, international study in order to compare the effectiveness of fingolimod versus dimethyl-fumarate on patients with Multiple Sclerosis. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2017-000559-26-IT.
EUCTR2020‐001205‐23‐SE {published data only}
-
- Ocrelizumab VErsus Rituximab off-Label at the Onset of Relapsing MS Disease. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2020-001205-23-SE.
EUCTR2020‐004505‐32‐FR {published data only}
-
- Study of efficacy and tolerability of ofatumumab vs. First Line disease modifying treatment (DMT) - physician’s choice in the treatment of newly diagnosed relapsing multiple sclerosis (RMS) patients. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2020-004505-32-FR.
EVOLVE‐MS‐1 Study {published data only}
-
- Wray S, Then Bergh F, Wundes A, Arnold DL, Drulovic J, Jasinska E, Bowen JD, Negroski D, Naismith RT, Hunter SF, Gudesblatt M, Chen H, Lyons J, Shankar SL, Kapadia S, Mendoza JP, Singer BA. Efficacy and Safety Outcomes with Diroximel Fumarate After Switching from Prior Therapies or Continuing on DRF: Results from the Phase 3 EVOLVE-MS-1 Study. Adv Ther 2022;39(4):1810-1831. [DOI: 10.1007/s12325-022-02068-7] [PMID: ] - DOI - PMC - PubMed
Masjedi 2021 {published data only}
NCT04695080 {published data only}
-
- ChariotMS - Cladribine to Halt Deterioration in People With Advanced Multiple Sclerosis (ChariotMS). https://clinicaltrials.gov/show/NCT04695080.
OPTIMUM {published data only}
-
- Diener, H C. The randomized phase III OPTIMUM study. Ponesimod compared with teriflunomide in patients with relapsing-remitting multiple sclerosis. Arzneimitteltherapie 2021;39(9):309‐310. [CENTRAL: CN-02343138]
RIFUND‐MS {published data only}
-
- Svenningsson A, Frisell T, Burman J, Salzer J, Fink K, Hallberg S, Hambraeus J, Axelsson M, Nimer FA, Sundström P, Gunnarsson M, Johansson R, Mellergård J, Rosenstein I, Ayad A, Sjöblom I, Risedal A, Flon P, Gilland E, Lindeberg J, Shawket F, Piehl F, Lycke J. Safety and efficacy of rituximab versus dimethyl fumarate in patients with relapsing-remitting multiple sclerosis or clinically isolated syndrome in Sweden: a rater-blinded, phase 3, randomised controlled trial. Lancet Neurol 2022;21(8):693-703. [DOI: 10.1016/S1474-4422(22)00209-5] [PMID: ] - DOI - PubMed
SUNBEAM/RADIANCE {published data only}
-
- Harris S, Comi G, Cree BAC, Arnold DL, Steinman L, Sheffield JK, Southworth H, Kappos L, Cohen JA, Ozanimod Study Investigators. Plasma neurofilament light chain concentrations as a biomarker of clinical and radiologic outcomes in relapsing multiple sclerosis: Post hoc analysis of Phase 3 ozanimod trials. Eur J Neurol 2021;28(11):3722-3730. [DOI: 10.1111/ene.15009] [PMID: ] - DOI - PMC - PubMed
References to ongoing studies
EUCTR2012‐000540‐10‐PL {published data only}
-
- Euctr P L. International clinical trial to compare ponesimod and teriflunomide in relapsing multiple sclerosis. http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2012-000540-10-PL 2015.
EUCTR2012‐003647‐30‐SK {published data only}
-
- Euctr S K. A clinical study in subjects with relapsing-remitting multiple sclerosis (RRMS) consisting of two parts: first part is to assess the efficacy, safety and tolerability of two oral doses of laquinimod either of 0.6 mg/day or 1.2mg/day (experimental drug) as compared placebo. Second part (all subjects receiving active treatment) is to evaluate the efficacy, safety and tolerability of two oral doses of laquinimod 0.6 mg/day or 1.2 mg/day (experimental drug). http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2012-003647-30-SK 2014.
EUCTR2013‐002082‐19‐SE {published data only}
-
- Euctr S E. A clinical study in subjects with relapsing-remitting multiple sclerosis (RRMS) to assess the efficacy, safety and tolerability of two oral doses of laquinimod either of 0.6 mg/day or 1.2mg/day (experimental drug) as compared to Interferon ß-1a (Avonex, authorised drug) administered once weekly. http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013-002082-19-SE 2013.
EUCTR2013‐003884‐71‐BE {published data only}
-
- EUCTR2013-003884-71-BE, Genzyme Corporation Yes. Phase IIIB-IV long term follow-up study for patients who participated in CAMMS03409. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2013-003884-71-BE 2014.
EUCTR2014‐001012‐19‐NL {published data only}
-
- EUCTR2014-001012-19-NL, Yes V U University Medical Center. Effects of fingolimod on advanced brain measures and clinical measures in multiple sclerosis. https://trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2014-001012-19-NL 2014.
EUCTR2018‐000284‐93‐BG {published data only}
-
- EUCTR2018-000284-93-BG, Mapi Pharma Ltd Yes. A multinational, multicenter, randomized, Phase III, double blind, parallel group, placebo controlled study in subjects with Relapsing forms of Multiple Sclerosis (RMS) to assess the efficacy, safety and tolerability of GA Depot, a long acting IM injection of glatiramer acetate, administered once every four weeks. https://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_nu... 2019.
EUCTR2018‐005038‐39‐GB {published data only}
-
- Euctr G B. A phase 2b study of Cladribine to halt deterioration in people with advanced multiple sclerosis. http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2018-005038-39-GB 2020.
EUCTR2019‐001505‐24‐NO {published data only}
-
- Euctr N O. A Clinical Study Comparing Rituximab and Cladribine for Relapsing Multiple Sclerosis. http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2019-001505-24-NO 2019.
EUCTR2020‐002981‐15‐DK {published data only}
-
- Euctr D K. Non-inferiority study of ocrelizumab and rituximab in active multiple sclerosis. http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2020-002981-15-DK 2020.
IRCT20130812014333N {published data only}
-
- Irct20130812014333N. Comparison of effectiveness and complication of rituximab and fingolimod in improvement disability motion. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20130812014333N125 2019.
IRCT201404195280N {published data only}
-
- Irct201404195280N. The therapeutic effect of Avonex, Rebif and Betaferon on disability and quality of life in multiple sclerosis: a Randomized Clinical Trial. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201404195280N16 2014.
NCT01404117 {published data only}
-
- Industries Teva Pharmaceutical. A Multinational, Randomized, Double-blind, Parallel-group, Placebo-controlled Study Assessing the Safety and Tolerability. https://ClinicalTrials.gov/show/NCT01404117 2012.
NCT01941004 {published data only}
-
- Nct. Safety and Efficacy of Fingolimod in MS Patients in China. https://clinicaltrials.gov/show/NCT01941004 2013.
NCT01975298 {published data only}
-
- NCT01975298. A Study to Evaluate 2 Doses Of Oral Administration Of Laquinimod Compared to Interferon ß-1a Administered by Injection in Participants With Relapsing Remitting Multiple Sclerosis (RRMS). https://clinicaltrials.gov/show/NCT01975298 2013.
NCT04056897 {published data only}
-
- Nct. Comparative Study of the Efficacy and Safety of BCD-132 With Teriflunomide and Placebo in Multiple Sclerosis. https://clinicaltrials.gov/show/NCT04056897 2019.
NCT04121221 {published data only}
-
- Nct. A Study to Asses Efficacy, Safety and Tolerability of Monthly Long-acting IM Injection of GA Depot in Subjects With RMS. https://clinicaltrials.gov/show/NCT04121221 2019.
NCT04121403 {published data only}
-
- Nct. Norwegian Study of Oral Cladribine and Rituximab in Multiple Sclerosis (NOR-MS). https://clinicaltrials.gov/show/NCT04121403 2019.
NCT04578639 {published data only}
-
- Nct. Ocrelizumab VErsus Rituximab Off-Label at the Onset of Relapsing MS Disease. https://clinicaltrials.gov/show/NCT04578639 2020.
NCT04688788 {published data only}
-
- Nct. Non-inferiority Study of Ocrelizumab and Rituximab in Active Multiple Sclerosis. https://clinicaltrials.gov/show/NCT04688788 2020.
WHO‐ICTRP 002519 {published data only}
-
- Study to evaluate the safety and efficacy of NU100 in patients with relapsing forms of multiple sclerosis. http://www.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2012/03/002519 2012.
WHO‐ICTRP PER‐024‐14 {published data only}
-
- Per. A randomized, double-blind, double-dummy, parallel-group study to evaluate the efficacy and safety of ocrelizumab in comparison to interferon beta-1a (rebif®) in patients with relapsing multiple sclerosis. http://www.who.int/trialsearch/Trial2.aspx?TrialID=PER-024-14 2014.
Additional references
Aharoni 2014
Alonso 2008
Alonso‐Coello 2016
-
- Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al, GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ 2016;353:i2016. [DOI: 10.1136/bmj.i2016] [PMID: ] - DOI - PubMed
Alonso‐Coello 2016b
-
- Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al, GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ 2016;353:i2089. [DOI: 10.1136/bmj.i2089] - DOI - PubMed
Association of British Neurologists 2005
-
- Association of British Neurologists 2005. Guidelines for the use of intravenous immunoglobulin in neurological diseases. www.theabn.org/documents/IVIg-Guidelines (accessed October 2013).
Awad 2009
Barten 2002
-
- Barten MJ, Gelder T, Gummert JF, Shorthouse R, Morris RE. Novel assays of multiplelymphocyte functions in whole blood measure: new mechanisms of actionof mycophenolate mofetil in vivo. Transpl Immunol 2002 Jun;10(1):1–14. - PubMed
Behrangi 2019
Benedict 2017
-
- Benedict RH, DeLuca J, Phillips G, LaRocca N, Hudson LD, Rudick R. Multiple Sclerosis Outcome Assessments Consortium. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis.. Mult Scler. 2017;23:721-733. [DOI: 10.1177/1352458517690821] - DOI - PMC - PubMed
Berntsson 2018
-
- Berntsson SG, Kristoffersson A, Boström I, Feresiadou A, Burman J, Landtblom AM. Rapidly increasing off-label use of rituximab in multiple sclerosis in Sweden - Outlier or predecessor? Acta Neurologica Scandinavica 2018;138(4):327-31. - PubMed
Beutler 1992
Brancati 2021
Brignardello‐Petersen 2018
-
- Brignardello-Petersen R, Bonner A, Alexander PE, Siemieniuk RA, Furukawa TA, Rochwerg B, et al, GRADE Working Group. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. Journal of Clinical Epidemiology 2018;93:36-44. - PubMed
Caldwell 2005
Carroll 2014
-
- Carroll CA, Fairman KA, Lage MJ. Updated cost-of-care estimates for commercially insured patients with multiple sclerosis: retrospective observational analysis of medical and pharmacy claims data. BMC Health Services Research 2014;14:286. [http://www.biomedcentral.com/1472-6963/14/286] - PMC - PubMed
Chaimani 2012
-
- Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161-76. - PubMed
Chaimani 2013
Chataway 2021
Ciccone 2008
Claussen 2012
-
- Claussen MC, Korn T. Immune mechanisms of new therapeutic strategies in MS: teriflunomide. Clinical Immunology 2012;142:49-56. - PubMed
Compston 2008
-
- Compston A, Coles A. Multiple sclerosis. Lancet 2008;372:1502-17. - PubMed
Da Costa 2013
-
- Da Costa BR, Nuesch E, Rutjes AW, Johnston BC, Reichenbach S, Trelle S, et al. Combining follow-up and change data is valid in meta-analyses of continuous outcomes: a meta-epidemiological study. Journal of Clinical Epidemiology 2013;66:847-55. - PubMed
Daruwalla 2023
-
- Daruwalla C, Shaygannejad V, Ozakbas S, Havrdova EK, Horakova D, Alroughani R, et al. Early non-disabling relapses are important predictors of disability accumulation in people with relapsing-remitting multiple sclerosis. Mult Scler 2023;13524585231151951:Epub ahead of print. [DOI: 10.1177/13524585231151951] - DOI - PMC - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177-88. - PubMed
Elovaara 2008
-
- Elovaara I, Apostolski S, Doorn P, Gilhus N, Hietaharju A, Honkaniemi J, et al. EFNS guidelines for the use of intravenous immunoglobulin in treatment of neurological diseases: EFNS task force on the use of intravenous immunoglobulin in treatment of neurological diseases. European Journal of Neurology 2008;15(9):893-908. - PubMed
EMA 2006
-
- European Agency for the Evaluation of Medicinal Products. Committee for proprietary medicinal products European public assessment report: Tysabri. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici... (accessed June 2014).
EMA 2011
-
- European Medicines Agency. Committee for proprietary medicinal products European public assessment report: Gilenya. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici... (accessed June 2014).
EMA 2013a
-
- European Medicines Agency. Committee for proprietary medicinal products European public assessment report: Aubagio. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici... (accessed June 2014).
EMA 2013b
-
- European Medicines Agency. Committee for proprietary medicinal products European public assessment report: Lemtrada. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info... (accessed June 2014).
EMA 2014a
-
- European Medicines Agency. Committee for proprietary medicinal products European public assessment report: Tecfidera. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info... (accessed June 2014).
EMA 2014b
-
- European Medicines Agency. Committee for proprietary medicinal products European public assessment report: Plegridy. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_... (accessed June 2015).
EMA 2014c
-
- European Medicines Agency. Refusal of the marketing authorisation for Nerventra (laquinimod). http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_... (accessed June 2014).
EMA 2017
-
- European Medicines Agency. Mavenclad. www.ema.europa.eu/en/medicines/human/EPAR/mavenclad.
EMA 2018
-
- European Medicines Agency. EMA recommends immediate suspension and recall of multiple sclerosis medicine Zinbryta. EMA Press Release 2018.
EMA 2018a
-
- European Medicines Agency. EMA recommends immediate suspension and recall of multiple sclerosis medicine Zinbryta. www.ema.europa.eu/en/news/ema-recommends-immediate-suspension-recall-mul....
EMA 2018b
-
- European Medicines Agency. Ocrevus. www.ema.europa.eu/en/medicines/human/EPAR/ocrevus.
EMA 2020
-
- European Medicines Agency. Mayzent. www.ema.europa.eu/en/medicines/human/EPAR/mayzent.
EMA 2021a
-
- European Medicines Agency. Zeposia. www.ema.europa.eu/en/medicines/human/EPAR/zeposia.
EMA 2021b
-
- European Medicines Agency. Ponvory. www.ema.europa.eu/en/medicines/human/EPAR/ponvory.
EMA 2021c
-
- European Medicines Agency. Vumerity. www.ema.europa.eu/en/medicines/human/EPAR/vumerity.
EMA 2021d
-
- European Medicines Agency. Kesimpta. www.ema.europa.eu/en/medicines/human/EPAR/kesimpta.
EMEA 1997
-
- European Agency for the Evaluation of Medicinal Products. Committee for proprietary medicinal products European public assessment report: Avonex. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_... (accessed June 2015).
EMEA 1998
-
- European Agency for the Evaluation of Medicinal Products. Committee for proprietary medicinal products European public assessment report: Rebif. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_R... (accessed June 2014).
EMEA 2002
-
- European Agency for the Evaluation of Medicinal Products. Committee for proprietary medicinal products European public assessment report: Betaferon. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici... (accessed June 2014).
FDA 1993
-
- US Food and Drug Administration. Betaseron interferon beta-1b subcutaneous. Drug Approval Package - Licensing Action 1993. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/103471s5063s506... (accessed June 2014).
FDA 1996
-
- US Food and Drug Administration. Glatiramer acetate (Capoxane) Product Approval Information - Licensing Action 1996. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseacti... (accessed June 2014).
FDA 2000
-
- US Food and Drug Administration. Mitoxantrone (Novantrone) Product Approval Information - Licensing Action 2000. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseacti... (accessed June 2014).
FDA 2002
-
- US Food and Drug Administration. Interferon beta-1a (Rebif) Product Approval Information - Licensing Action 2002. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDeveloped... (accessed June 2014).
FDA 2003
-
- US Food and Drug Administration. Interferon beta-1a (Avonex) Product Approval Information - Licensing Action 2003. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/103628s5021TOC.cfm (accessed June 2014).
FDA 2004
-
- US Food and Drug Administration. Tysabri (Natalizumab) Product Approval Information 2004. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/125104s000_Natali... (accessed June 2014).
FDA 2006
-
- US Food and Drug Administration. FDA Approves Resumed Marketing of Tysabri Under a Special Distribution Program. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108662... (accessed June 2014).
FDA 2010
-
- US Food and Drug Administration. Gilenya (Fingolimod) Product Approval Information 2010. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseacti... (accessed June 2014).
FDA 2012
-
- US Food and Drug Administration. Aubagio (Teriflunomide) Product Approval Information 2012. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseacti... (accessed June 2014).
FDA 2013
-
- US Food and Drug Administration. Tecfidera (Dimethyl fumarate) Product Approval Information 2013. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseacti... (accessed June 2014).
FDA 2014a
-
- US Food and Drug Administration. Alemtuzumab (Lemtrada) Product Approval Information. Licensing Action 2014. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2014/103948Orig1... (accessed Sept 2014).
FDA 2014b
-
- US Food and Drug Administration. Peginterferon beta-1a (Plegridy) Product Approval Information. Licensing Action 2014. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/biologics/2004/1... (accessed September 2014).
FDA 2014c
-
- US Food and Drug Administration. Imuran Product Information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/016324s037,0173... (accessed June 2015).
FDA 2017
-
- US Food and Drug Administration. FDA approves new drug to treat multiple sclerosis. www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treat-... (accessed March 2017).
FDA 2018
-
- US Food and Drug Administration. FDA working with manufacturers to withdraw Zinbryta from the market in the United States. www.fda.gov/drugs/drug-safety-and-availability/fda-working-manufacturers... (accessed March 2018).
FDA 2019a
-
- US Food and Drug Administration. FDA approves new oral treatment for multiple sclerosis. www.fda.gov/news-events/press-announcements/fda-approves-new-oral-treatm... (accessed April 2019).
FDA 2019b
-
- US Food and Drug Administration. FDA approves new oral drug to treat multiple sclerosis. www.fda.gov/news-events/press-announcements/fda-approves-new-oral-drug-t... (accessed April 2019).
FDA 2020
-
- US Food and Drug Administration. Novel drug approvals for 2020. www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-the... (accessed Jan 2021).
FDA 2021
-
- US Food and Drug Administration. Novel Drug Approvals for 2021. www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-the... May 2023).
Filippi 2018
Filippini 2013
Fogarty 2016
Fox 2004
-
- Fox E. Mechanism of action of mitoxantrone. Neurology 2004;12:15-8. - PubMed
Frohman 2004
Glenny 2005
-
- Glenny A, Altman D, Song F, Sakarovitch C, Deeks J, D'Amico R, et al. Indirect comparisons of competing interventions. Health Technology Assessment 2005;9:1-34. - PubMed
GRADEpro 2008 [Computer program]
-
- GRADEpro. Version 3.2 for Windows. Brozek J, Oxman A, Schünemann H, 2008.
Greenberg 2016
Gronwall 1977
Guyatt 2011
Guyatt 2013
Haider 2021
Hauser 2020
Hawton 2016
He 2020
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2012
Higgins 2021
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Hill‐Cawthorne 2012
-
- Hill-Cawthorne GA, Button T, Tuohy O, Jones JL, May K, Somerfield J, et al. Long term lymphocyte reconstitution after alemtuzumab treatment of multiple sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry 2012;83:298-304. - PubMed
Hu 2012
-
- Hu X, Miller L, Richman S, Hitchman S, Glick G, Liu S, et al. A novel PEGylated interferon beta-1a for multiple sclerosis: safety, pharmacology, and biology. Journal of Clinical Pharmacology 2012;52:798–808. - PubMed
Hultcrantz 2017
Jackson 2014
Kang 2021
Kieseier 2011
-
- Kieseier BC. The mechanism of action of interferon-β in relapsing multiple sclerosis. CNS Drugs 2011;25:491-502. - PubMed
Koch‐Henriksen 2021
Korteweg 2006
Kurtzke 1983
-
- Kurtzke J. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444-52. - PubMed
Laurson‐Doube 2021
Leist 2011
-
- Leist TP, Weissert R. Cladribine: mode of action and implications for treatment of multiple sclerosis. Clinical Neuropharmacology 2011;34(1):28-35. [PMID: ] - PubMed
Li 2020
Linker 2011
-
- Linker RA, Lee DH, Ryan S, Van Dam AM, Conrad R, Bista P, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011;134(3):678-92. - PubMed
Liu 2021
Love 2002
-
- Love R. Potential antibiotic treatment for multiple sclerosis? Lancet 2002;359:50. [DOI: 10.1016/S0140-6736(02)07248-3] - DOI
Lublin 1996
Lublin 2014
Lucchetta 2018
Lucchetti 2018
-
- Luchetti S, Fransen NL, Eden CG, Ramaglia V, Mason M, Huitinga I. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol 2018;135:511-28. [DOI: 10.1007/s00401-018-1818-y] - DOI - PMC - PubMed
Mandala 2002
-
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 2002;296:346-9. - PubMed
Massacesi 2002
-
- Massacesi L. Compartmentalization of the immune response in the central nervous system and natural history of multiple sclerosis. Implications for therapy. Clinical Neurology and Neurosurgery 2002;104(3):177-81. - PubMed
McDonald 2001
-
- McDonald W, Compston A, Edan G, Goodkin D, Hartung H, Lublin F. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Annals of Neurology 2001;50:121-7. - PubMed
Meinl 2008
-
- Meinl E, Krumbholz M, Derfuss T, Junker A, Hohlfeld R. Compartmentalization of inflammation in the CNS: a major mechanism driving progressive multiple sclerosis. Journal of the Neurological Sciences 2008;274(1-2):42-4. - PubMed
Metz 2017
Miladinovic 2014
-
- Miladinovic B, Hozo I, Chaimani A, Djulbegovic B. Indirect treatment comparison. Stata Journal 2014;14(1):76-86.
Morgano 2022
-
- Morgano GP, Mbuagbaw L, Santesso N, Xie F, Brozek JL, Siebert U, et al. Defining decision thresholds for judgments on health benefits and harms using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Evidence to Decision (EtD) frameworks: a protocol for a randomised methodological study (GRADE-THRESHOLD). BMJ Open 2022;12(3):e053246. - PMC - PubMed
Oh 2013
-
- Oh J, Calabresi PA. Emerging injectable therapies for multiple sclerosis. Lancet Neurology 2013;12:1115–26. - PubMed
Peters 2008
-
- Peters J, Sutton A, Jones D, Abrams K, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991-6. - PubMed
Polman 2005
-
- Polman C, Reingold S, Edan G, Filippi M, Hartung H, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the 'McDonald Criteria'. Annals of Neurology 2005;58:840-6. - PubMed
Polman 2011
Poser 1983
-
- Poser C, Paty D, Scheinberg L, McDonald W, Davis F, Ebers G, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;13:227-31. - PubMed
Ruggieri 2022
Salanti 2011
-
- Salanti G, Ades A, Ioannidis J. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64:163-71. - PubMed
Salanti 2012
-
- Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80-97. - PubMed
Salzer 2016
Scalfari 2014
-
- Scalfari A, Neuhaus A, Daumer M, Muraro PA, Ebers GC. Onset of secondary progressive phase and long-term evolution of multiple sclerosis. Journal of Neurology, Neurosurgery & Psychiatry 2014;85:67-75. - PubMed
Schmied 2003
-
- Schmied M, Duda PW, Krieger JI, Trollmo C, Hafler DA. In vitro evidence that subcutaneous administration of glatiramer acetate induces hyporesponsive T cells in patients with multiple sclerosis. Clinical Immunology 2003;106:163-74. - PubMed
Schünemann 2022a
-
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Schünemann 2022b
-
- Schünemann HJ, Neumann I, Hultcrantz M, Brignardello-Petersen R, Zeng L, Murad MH, et al. GRADE guidance 35: update on rating imprecision for assessing contextualized certainty of evidence and making decisions. Journal of Clinical Epidemiology 2022;150:225-42. - PubMed
Scott 2016
-
- Scott FL, Clemons B, Brooks J, Brahmachary E, Powell R, Dedman H, et al. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity. Br J Pharmacol 2016;173(11):1778-92. [DOI: 10.1111/bph.13476] - DOI - PMC - PubMed
Stangel 1999
-
- Stangel M, Toyka K, Gold R. Mechanisms of high-dose intravenous immunoglobulins in demyelinating diseases. Archives of Neurology 1999;56(6):661-3. - PubMed
Thompson 2018
Tiede 2003
Tramacere 2023
Tremlett 2008
Van Wijmeersch 2022
Varrin‐Doyer 2014
Veroniki 2013
White 2011
-
- White IR. Multivariate random-effects meta-regression: updates to mvmeta. Stata Journal 2011;11:255-70.
White 2012
Wilms 2010
-
- Wilms H, Sievers J, Rickert U, Rostami-Yazdi M, Mrowietz U, Lucius R. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1beta, TNF-alpha and IL-6 in an in-vitro model of brain inflammation. Journal of Neuroinflammation 2010;7:30. - PMC - PubMed
Wuest 2011
Yednock 1992
-
- Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature 1992;356:63-6. - PubMed
Yousry 2006
Zeineddine 2020
Zinzani 1994
-
- Zinzani PL, Buzzi M, Farabegoli P, Martinelli G, Tosi P, Zuffa E, Visani G, Testoni N, Salvucci M, Bendandi M, et al. Apoptosis induction with fludarabine on freshly isolated chronic myeloid leukemia cells. Haematologica 1994;79:127-31. - PubMed
References to other published versions of this review
Tramacere 2014
-
- Tramacere I, Del Giovane C, Salanti G, D'Amico R, Pacchetti I, Filippini G. Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis. Cochrane Database of Systematic Reviews 2014, Issue 11. Art. No: CD011381. [DOI: 10.1002/14651858.CD011381] - DOI - PMC - PubMed
Tramacere 2015
-
- Tramacere I, Del Giovane C, Salanti G, D'Amico R, Filippini G. Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD011381. [DOI: 10.1002/14651858.CD011381.pub2] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources


