Metformin induces M2 polarization via AMPK/PGC-1α/PPAR-γ pathway to improve peripheral nerve regeneration
- PMID: 37303686
- PMCID: PMC10250979
Metformin induces M2 polarization via AMPK/PGC-1α/PPAR-γ pathway to improve peripheral nerve regeneration
Abstract
Objectives: Investigating the effect of metformin on peripheral nerve regeneration and the molecular mechanism.
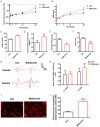
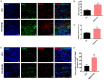
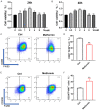
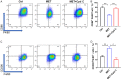
Methods: In this study, a rat model of sciatic nerve injury and an inflammatory bone marrow-derived macrophage (BMDM) cell model were established. We assessed the sensory and motor function of the hind limbs four weeks after sciatic nerve injury, immunofluorescence was used to detect axonal regeneration and myelin formation, as well as local macrophage subtypes. We investigated the polarizing effect of metformin on inflammatory macrophages, and western blotting was applied to detect the molecular mechanisms behind it.
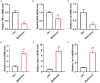
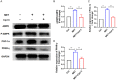
Results: Metformin treatment accelerated functional recovery, axon regeneration and remyelination, and promoted M2 macrophage polarization. In vivo, metformin transformed pro-inflammatory macrophages into pro-regeneration M2 macrophages. Protein expression levels of phosphorylated AMP-activated protein kinase (p-AMPK), proliferator-activated receptor-γ co-activator 1α (PGC-1α), and peroxisome proliferator-activated receptor-γ (PPAR-γ) increased upon metformin treatment. Moreover, inhibition of AMPK abolished the effects of metformin treatment on M2 polarization.
Conclusion: Metformin promoted M2 macrophage polarization by activating the AMPK/PGC-1α/PPAR-γ signaling axis, thereby promoting peripheral nerve regeneration.
Keywords: AMPK/PGC-1α/PPAR-γ signaling axis; Metformin; macrophage polarization; peripheral nerve regeneration.
AJTR Copyright © 2023.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Down-regulation miR-146a-5p in Schwann cell-derived exosomes induced macrophage M1 polarization by impairing the inhibition on TRAF6/NF-κB pathway after peripheral nerve injury.Exp Neurol. 2023 Apr;362:114295. doi: 10.1016/j.expneurol.2022.114295. Epub 2022 Dec 6. Exp Neurol. 2023. PMID: 36493861
-
Norisoboldine Attenuates Sepsis-Induced Acute Lung Injury by Modulating Macrophage Polarization via PKM2/HIF-1α/PGC-1α Pathway.Biol Pharm Bull. 2021;44(10):1536-1547. doi: 10.1248/bpb.b21-00457. Biol Pharm Bull. 2021. PMID: 34602563
-
Energy-sensing factors coactivator peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) and AMP-activated protein kinase control expression of inflammatory mediators in liver: induction of interleukin 1 receptor antagonist.J Biol Chem. 2012 Jan 13;287(3):1847-60. doi: 10.1074/jbc.M111.302356. Epub 2011 Nov 23. J Biol Chem. 2012. PMID: 22117073 Free PMC article.
-
Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways.Cell Signal. 2014 Feb;26(2):192-7. doi: 10.1016/j.cellsig.2013.11.004. Epub 2013 Nov 9. Cell Signal. 2014. PMID: 24219909 Review.
-
The Role of Macrophages in Nerve Regeneration: Polarization and Combination with Tissue Engineering.Tissue Eng Part B Rev. 2024 Jun 27. doi: 10.1089/ten.TEB.2024.0100. Online ahead of print. Tissue Eng Part B Rev. 2024. PMID: 38832865 Review.
Cited by
-
Transcriptional Regulation and Function of Malic Enzyme 1 in Human Macrophage Activation.Biomedicines. 2024 Sep 13;12(9):2089. doi: 10.3390/biomedicines12092089. Biomedicines. 2024. PMID: 39335602 Free PMC article.
-
Metformin impacts the differentiation of mouse bone marrow cells into macrophages affecting tumour immunity.Heliyon. 2024 Sep 11;10(18):e37792. doi: 10.1016/j.heliyon.2024.e37792. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39315158 Free PMC article.
-
Shaping the immune landscape: Multidimensional environmental stimuli refine macrophage polarization and foster revolutionary approaches in tissue regeneration.Heliyon. 2024 Aug 30;10(17):e37192. doi: 10.1016/j.heliyon.2024.e37192. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39296009 Free PMC article. Review.
-
O6-methylguanine DNA methyltransferase regulates β-glucan-induced trained immunity of macrophages via farnesoid X receptor and AMPK.iScience. 2023 Dec 14;27(1):108733. doi: 10.1016/j.isci.2023.108733. eCollection 2024 Jan 19. iScience. 2023. PMID: 38235325 Free PMC article.
-
The AMPK activator metformin improves recovery from demyelination by shifting oligodendrocyte bioenergetics and accelerating OPC differentiation.Front Cell Neurosci. 2023 Oct 12;17:1254303. doi: 10.3389/fncel.2023.1254303. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37904733 Free PMC article.
References
-
- Li J, Yao Y, Wang Y, Xu J, Zhao D, Liu M, Shi S, Lin Y. Modulation of the crosstalk between Schwann cells and macrophages for nerve regeneration: a therapeutic strategy based on a multifunctional tetrahedral framework nucleic acids system. Adv Mater. 2022;34:e2202513. - PubMed
LinkOut - more resources
Full Text Sources

