Recent advances in the delivery and applications of nonviral CRISPR/Cas9 gene editing
- PMID: 36988873
- PMCID: PMC10052255
- DOI: 10.1007/s13346-023-01320-z
Recent advances in the delivery and applications of nonviral CRISPR/Cas9 gene editing
Abstract
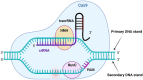
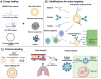
The CRISPR (clustered regularly interspaced short palindromic repeats)/Cas9 genome editing system has been a major technological breakthrough that has brought revolutionary changes to genome editing for therapeutic and diagnostic purposes and precision medicine. With the advent of the CRISPR/Cas9 system, one of the critical limiting factors has been the safe and efficient delivery of this system to cells or tissues of interest. Several approaches have been investigated to find delivery systems that can attain tissue-targeted delivery, lowering the chances of off-target editing. While viral vectors have shown promise for in vitro, in vivo and ex vivo delivery of CRISPR/Cas9, their further clinical applications have been restricted due to shortcomings including limited cargo packaging capacity, difficulties with large-scale production, immunogenicity and insertional mutagenesis. Rapid progress in nonviral delivery vectors, including the use of lipid, polymer, peptides, and inorganic nanoparticle-based delivery systems, has established nonviral delivery approaches as a viable alternative to viral vectors. This review will introduce the molecular mechanisms of the CRISPR/Cas9 gene editing system, current strategies for delivering CRISPR/Cas9-based tools, an overview of strategies for overcoming off-target genome editing, and approaches for improving genome targeting and tissue targeting. We will also highlight current developments and recent clinical trials for the delivery of CRISPR/Cas9. Finally, future directions for overcoming the limitations and adaptation of this technology for clinical trials will be discussed.
Keywords: CRISPR/Cas9; Clinical applications; Gene therapy; Gene-editing; Nonviral delivery system; Vectors.
© 2023. Controlled Release Society.
Conflict of interest statement
All authors declare no competing interests.
Figures
Similar articles
-
Delivery of Tissue-Targeted Scalpels: Opportunities and Challenges for In Vivo CRISPR/Cas-Based Genome Editing.ACS Nano. 2020 Aug 25;14(8):9243-9262. doi: 10.1021/acsnano.0c04707. Epub 2020 Jul 22. ACS Nano. 2020. PMID: 32697075 Free PMC article.
-
CRISPR/Cas9-Based Genome Editing for Disease Modeling and Therapy: Challenges and Opportunities for Nonviral Delivery.Chem Rev. 2017 Aug 9;117(15):9874-9906. doi: 10.1021/acs.chemrev.6b00799. Epub 2017 Jun 22. Chem Rev. 2017. PMID: 28640612 Review.
-
Gene Therapy with CRISPR/Cas9 Coming to Age for HIV Cure.AIDS Rev. 2017 Oct-Dec;19(3):167-172. AIDS Rev. 2017. PMID: 29019352
-
Pre-clinical non-viral vectors exploited for in vivo CRISPR/Cas9 gene editing: an overview.Biomater Sci. 2022 Jun 28;10(13):3410-3432. doi: 10.1039/d1bm01452h. Biomater Sci. 2022. PMID: 35604372 Review.
-
Lipid nanoparticles: The game-changer in CRISPR-Cas9 genome editing.Heliyon. 2024 Jan 11;10(2):e24606. doi: 10.1016/j.heliyon.2024.e24606. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38288017 Free PMC article. Review.
Cited by
-
Emerging Gene-editing nano-therapeutics for Cancer.Heliyon. 2024 Oct 20;10(21):e39323. doi: 10.1016/j.heliyon.2024.e39323. eCollection 2024 Nov 15. Heliyon. 2024. PMID: 39524822 Free PMC article. Review.
-
Leveraging CRISPR gene editing technology to optimize the efficacy, safety and accessibility of CAR T-cell therapy.Leukemia. 2024 Oct 25. doi: 10.1038/s41375-024-02444-y. Online ahead of print. Leukemia. 2024. PMID: 39455854 Review.
-
Nanotechnology and CRISPR/Cas-Mediated Gene Therapy Strategies: Potential Role for Treating Genetic Disorders.Mol Biotechnol. 2024 Oct 24. doi: 10.1007/s12033-024-01301-8. Online ahead of print. Mol Biotechnol. 2024. PMID: 39446301 Review.
-
Versatile Cell Penetrating Peptide for Multimodal CRISPR Gene Editing in Primary Stem Cells.bioRxiv [Preprint]. 2024 Sep 23:2024.09.23.614499. doi: 10.1101/2024.09.23.614499. bioRxiv. 2024. PMID: 39386541 Free PMC article. Preprint.
-
Potential Use of MicroRNA Technology in Thalassemia Therapy.J Clin Med Res. 2024 Sep;16(9):411-422. doi: 10.14740/jocmr5245. Epub 2024 Aug 22. J Clin Med Res. 2024. PMID: 39346566 Free PMC article. Review.
References
-
- Genetic Alliance. District of Columbia Department of Health Understanding genetics: a district of Columbia guide for patients and health professionals, in: Genetic Alliance Monographs and Guides. Washington (DC): Genetic Alliance; 2010. - PubMed
-
- Gillet J-P, Macadangdang B, Fathke RL, Gottesman MM, Kimchi-Sarfaty C. The development of gene therapy: from monogenic recessive disorders to complex diseases such as cancer. In: Gene Therapy of Cancer. 2009. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources


