Smoking-Related Interstitial Fibrosis (SRIF) in Patients Presenting With Diffuse Parenchymal Lung Disease
- PMID: 36495281
- PMCID: PMC9891418
- DOI: 10.1093/ajcp/aqac144
Smoking-Related Interstitial Fibrosis (SRIF) in Patients Presenting With Diffuse Parenchymal Lung Disease
Abstract
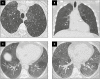
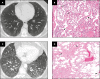
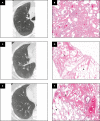
Objectives: To describe the clinical, radiologic, and pathologic findings in cases where smoking-related interstitial fibrosis (SRIF) was diagnosed in surgical lung biopsy specimens from patients with clinical and imaging features of diffuse parenchymal lung disease (DPLD).
Methods: Cases were included in this study if patients had clinical and imaging evidence of DPLD and surgical lung biopsy specimens revealed SRIF. A dedicated multidisciplinary conference was held to correlate clinical, radiologic, and pathologic findings.
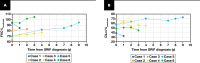
Results: Six cases met inclusion criteria; all six (five women/one man, aged 42-57 years, mean age 47 years) were either current smokers (five of six) or ex-smokers (one of six) and were evaluated for respiratory symptoms and abnormal pulmonary function tests, most commonly reduced forced vital capacity (n = 3) and diffusing capacity for carbon monoxide (n = 6). The most common imaging abnormalities were bilateral ground-glass opacities, which correlated with histopathologic SRIF. Follow-up of up to 10 years showed stable or improved clinical symptoms, pulmonary function tests, and radiologic findings with smoking cessation (three patients) or a decrease in smoking (three patients). No specific treatments were given, and those treated with empiric corticosteroid tapers did not show discernible responses.
Conclusions: SRIF can present as clinically meaningful diffuse parenchymal lung disease in relatively young heavy smokers, characterized by bilateral ground-glass opacities and a stable clinical course.
Keywords: Desquamative interstitial pneumonia; Diffuse parenchymal lung disease; Interstitial lung disease; Respiratory bronchiolitis; Smoking-related interstitial fibrosis.
© American Society for Clinical Pathology, 2022.
Figures


Similar articles
-
Smoking-Related Interstitial Fibrosis and Smoker's Macrophages.J Nippon Med Sch. 2024 Mar 9;91(1):20-27. doi: 10.1272/jnms.JNMS.2024_91-113. Epub 2024 Jan 16. J Nippon Med Sch. 2024. PMID: 38233126
-
Smoking-Related Interstitial Fibrosis: Evidence of Radiologic Regression with Advancing Age and Smoking Cessation.COPD. 2017 Dec;14(6):603-609. doi: 10.1080/15412555.2017.1378631. Epub 2017 Oct 18. COPD. 2017. PMID: 29043847
-
Respiratory bronchiolitis, respiratory bronchiolitis-associated interstitial lung disease, and desquamative interstitial pneumonia: different entities or part of the spectrum of the same disease process?AJR Am J Roentgenol. 1999 Dec;173(6):1617-22. doi: 10.2214/ajr.173.6.10584810. AJR Am J Roentgenol. 1999. PMID: 10584810
-
Smoking-Related Interstitial Lung Diseases.Immunol Allergy Clin North Am. 2023 May;43(2):273-287. doi: 10.1016/j.iac.2023.01.007. Epub 2023 Mar 1. Immunol Allergy Clin North Am. 2023. PMID: 37055089 Review.
-
Pathologic features of smoking-related lung diseases, with emphasis on smoking-related interstitial fibrosis and a consideration of differential diagnoses.Semin Diagn Pathol. 2018 Sep;35(5):315-323. doi: 10.1053/j.semdp.2018.08.002. Epub 2018 Aug 10. Semin Diagn Pathol. 2018. PMID: 30154023 Review.
References
-
- Katzenstein AL, Mukhopadhyay S, Zanardi C, et al. . Clinically occult interstitial fibrosis in smokers: classification and significance of a surprisingly common finding in lobectomy specimens. Hum Pathol. 2010;41:316-325. - PubMed
-
- Katzenstein AL. Smoking-related interstitial fibrosis (SRIF): pathologic findings and distinction from other chronic fibrosing lung diseases. J Clin Pathol. 2013;66:882-887. - PubMed
-
- Fabre A, Treacy A, Lavelle LP, et al. . Smoking-related interstitial fibrosis: evidence of radiologic regression with advancing age and smoking cessation. COPD. 2017;14:603-609. - PubMed
-
- Wick MR. Pathologic features of smoking-related lung diseases, with emphasis on smoking-related interstitial fibrosis, and a consideration of differential diagnoses. Semin Diagn Pathol. 2018;35:315-323. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous


