Pre- and Postlicensure Animal Efficacy Studies Comparing Anthrax Antitoxins
- PMID: 36251555
- PMCID: PMC9649416
- DOI: 10.1093/cid/ciac593
Pre- and Postlicensure Animal Efficacy Studies Comparing Anthrax Antitoxins
Abstract
Background: The deliberate use of Bacillus anthracis spores is believed by the US government to be a high bioweapons threat. The first line of defense following potential exposure to B. anthracis spores would be postexposure prophylaxis with antimicrobials that have activity against B. anthracis. Additional therapies to address the effects of toxins may be needed in systemically ill individuals. Over the last 2 decades, the United States government (USG) collaborated with the private sector to develop, test, and stockpile 3 antitoxins: anthrax immunoglobulin intravenous (AIGIV), raxibacumab, and obiltoxaximab. All 3 products target protective antigen, a protein factor common to the 2 exotoxins released by B. anthracis, and hamper or block the toxins' effects and prevent or reduce pathogenesis. These antitoxins were approved for licensure by the United States Food and Drug Administration based on animal efficacy studies compared to placebo.
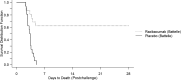
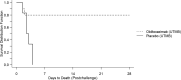
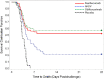
Methods: We describe USG-sponsored pre- and postlicensure studies that compared efficacy of 3 antitoxins in a New Zealand White rabbit model of inhalation anthrax; survival following a lethal aerosolized dose of B. anthracis spores was the key measure of effectiveness. To model therapeutic intervention, intravenous treatments were started following onset of antigenemia.
Results: In pre- and postlicensure studies, all 3 antitoxins were superior to placebo; in the postlicensure study, raxibacumab and obiltoxaximab were superior to AIGIV, but neither was superior to the other.
Conclusions: These data illustrate the relative therapeutic benefit of the 3 antitoxins and provide a rationale to prioritize their deployment.
Keywords: Bacillus anthracis; Inhalation anthrax; New Zealand white rabbit; antitoxin; protective antigen.
Published by Oxford University Press on behalf of Infectious Diseases Society of America 2022. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures
Similar articles
-
Development of Protective Immunity in New Zealand White Rabbits Challenged with Bacillus anthracis Spores and Treated with Antibiotics and Obiltoxaximab, a Monoclonal Antibody against Protective Antigen.Antimicrob Agents Chemother. 2018 Jan 25;62(2):e01590-17. doi: 10.1128/AAC.01590-17. Print 2018 Feb. Antimicrob Agents Chemother. 2018. PMID: 29133571 Free PMC article.
-
Obiltoxaximab Prevents Disseminated Bacillus anthracis Infection and Improves Survival during Pre- and Postexposure Prophylaxis in Animal Models of Inhalational Anthrax.Antimicrob Agents Chemother. 2016 Sep 23;60(10):5796-805. doi: 10.1128/AAC.01102-16. Print 2016 Oct. Antimicrob Agents Chemother. 2016. PMID: 27431219 Free PMC article.
-
A Review of the Efficacy of FDA-Approved B. anthracis Anti-Toxin Agents When Combined with Antibiotic or Hemodynamic Support in Infection- or Toxin-Challenged Preclinical Models.Toxins (Basel). 2021 Jan 13;13(1):53. doi: 10.3390/toxins13010053. Toxins (Basel). 2021. PMID: 33450877 Free PMC article. Review.
-
A high-affinity monoclonal antibody to anthrax protective antigen passively protects rabbits before and after aerosolized Bacillus anthracis spore challenge.Infect Immun. 2005 Feb;73(2):795-802. doi: 10.1128/IAI.73.2.795-802.2005. Infect Immun. 2005. PMID: 15664918 Free PMC article.
-
Obiltoxaximab: First Global Approval.Drugs. 2016 May;76(7):823-30. doi: 10.1007/s40265-016-0577-0. Drugs. 2016. PMID: 27085536 Review.
Cited by
-
Anthrax: A narrative review.New Microbes New Infect. 2024 Oct 10;62:101501. doi: 10.1016/j.nmni.2024.101501. eCollection 2024 Dec. New Microbes New Infect. 2024. PMID: 39497912 Free PMC article. Review.
-
Single domain antibodies from camelids in the treatment of microbial infections.Front Immunol. 2024 May 17;15:1334829. doi: 10.3389/fimmu.2024.1334829. eCollection 2024. Front Immunol. 2024. PMID: 38827746 Free PMC article. Review.
-
CDC Guidelines for the Prevention and Treatment of Anthrax, 2023.MMWR Recomm Rep. 2023 Nov 17;72(6):1-47. doi: 10.15585/mmwr.rr7206a1. MMWR Recomm Rep. 2023. PMID: 37963097 Free PMC article.
-
Human Anthrax: Update of the Diagnosis and Treatment.Diagnostics (Basel). 2023 Mar 10;13(6):1056. doi: 10.3390/diagnostics13061056. Diagnostics (Basel). 2023. PMID: 36980364 Free PMC article. Review.
-
Responding to the Threat Posed by Anthrax: Updated Evidence to Improve Preparedness.Clin Infect Dis. 2022 Oct 17;75(Suppl 3):S339-S340. doi: 10.1093/cid/ciac567. Clin Infect Dis. 2022. PMID: 36251547 Free PMC article. No abstract available.
References
-
- Davies JC. A major epidemic of anthrax in Zimbabwe. The experience at the Beatrice Road Infectious Diseases Hospital, Harare. Cent Afr J Med 1985; 31:176–180. - PubMed
-
- Beatty ME, Ashford DA, Griffin PM, Tauxe RV, Sobel J. Gastrointestinal anthrax: review of the literature. Arch Intern Med 2003; 163:2527–31. - PubMed
-
- Holty J-EC, Bravata DM, Liu H, Olshen RA, McDonald KM, Owens DK. Systematic review: a century of inhalational anthrax cases from 1900 to 2005. Ann Intern Med 2006; 144:270–80. - PubMed
-
- Moayeri M, Leppla SH, Vrentas C, Pomerantsev A, Liu S. Anthrax pathogenesis. Ann Rev Microbiol 2015; 69:185–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical


