Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination
- PMID: 35857583
- PMCID: PMC9348751
- DOI: 10.1126/sciimmunol.add4853
Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination
Abstract
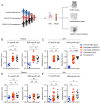
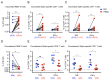
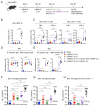
SARS-CoV-2 mRNA vaccination induces robust humoral and cellular immunity in the circulation; however, it is currently unknown whether it elicits effective immune responses in the respiratory tract, particularly against variants of concern (VOCs), including Omicron. We compared the SARS-CoV-2 S-specific total and neutralizing antibody responses, and B and T cell immunity, in the bronchoalveolar lavage fluid (BAL) and blood of COVID-19-vaccinated individuals and hospitalized patients. Vaccinated individuals had significantly lower levels of neutralizing antibody against D614G, Delta (B.1.617.2), and Omicron BA.1.1 in the BAL compared with COVID-19 convalescents despite robust S-specific antibody responses in the blood. Furthermore, mRNA vaccination induced circulating S-specific B and T cell immunity, but in contrast to COVID-19 convalescents, these responses were absent in the BAL of vaccinated individuals. Using a mouse immunization model, we demonstrated that systemic mRNA vaccination alone induced weak respiratory mucosal neutralizing antibody responses, especially against SARS-CoV-2 Omicron BA.1.1 in mice; however, a combination of systemic mRNA vaccination plus mucosal adenovirus-S immunization induced strong neutralizing antibody responses not only against the ancestral virus but also the Omicron BA.1.1 variant. Together, our study supports the contention that the current COVID-19 vaccines are highly effective against severe disease development, likely through recruiting circulating B and T cell responses during reinfection, but offer limited protection against breakthrough infection, especially by the Omicron sublineage. Hence, mucosal booster vaccination is needed to establish robust sterilizing immunity in the respiratory tract against SARS-CoV-2, including infection by the Omicron sublineage and future VOCs.
Figures

Comment in
-
Operation Nasal Vaccine-Lightning speed to counter COVID-19.Sci Immunol. 2022 Aug 12;7(74):eadd9947. doi: 10.1126/sciimmunol.add9947. Epub 2022 Aug 19. Sci Immunol. 2022. PMID: 35862488
Similar articles
-
Vaccination and Omicron BA.1/BA.2 Convalescence Enhance Systemic but Not Mucosal Immunity against BA.4/5.Microbiol Spectr. 2023 Jun 15;11(3):e0516322. doi: 10.1128/spectrum.05163-22. Epub 2023 Apr 26. Microbiol Spectr. 2023. PMID: 37098903 Free PMC article.
-
Intranasal SARS-CoV-2 Omicron variant vaccines elicit humoral and cellular mucosal immunity in female mice.EBioMedicine. 2024 Jul;105:105185. doi: 10.1016/j.ebiom.2024.105185. Epub 2024 Jun 7. EBioMedicine. 2024. PMID: 38848648 Free PMC article.
-
Pre-Omicron Vaccine Breakthrough Infection Induces Superior Cross-Neutralization against SARS-CoV-2 Omicron BA.1 Compared to Infection Alone.Int J Mol Sci. 2022 Jul 12;23(14):7675. doi: 10.3390/ijms23147675. Int J Mol Sci. 2022. PMID: 35887023 Free PMC article.
-
Defending against SARS-CoV-2: The T cell perspective.Front Immunol. 2023 Jan 27;14:1107803. doi: 10.3389/fimmu.2023.1107803. eCollection 2023. Front Immunol. 2023. PMID: 36776863 Free PMC article. Review.
-
B-cell and antibody responses to SARS-CoV-2: infection, vaccination, and hybrid immunity.Cell Mol Immunol. 2024 Feb;21(2):144-158. doi: 10.1038/s41423-023-01095-w. Epub 2023 Nov 10. Cell Mol Immunol. 2024. PMID: 37945737 Free PMC article. Review.
Cited by
-
Intranasal delivery of a subunit protein vaccine provides protective immunity against JN.1 and XBB-lineage variants.Signal Transduct Target Ther. 2024 Nov 20;9(1):311. doi: 10.1038/s41392-024-02025-6. Signal Transduct Target Ther. 2024. PMID: 39562542 Free PMC article.
-
T-Cell Immune Responses to SARS-CoV-2 Infection and Vaccination.Vaccines (Basel). 2024 Sep 30;12(10):1126. doi: 10.3390/vaccines12101126. Vaccines (Basel). 2024. PMID: 39460293 Free PMC article. Review.
-
Navigating the Landscape of B Cell Mediated Immunity and Antibody Monitoring in SARS-CoV-2 Vaccine Efficacy: Tools, Strategies and Clinical Trial Insights.Vaccines (Basel). 2024 Sep 24;12(10):1089. doi: 10.3390/vaccines12101089. Vaccines (Basel). 2024. PMID: 39460256 Free PMC article. Review.
-
SARS-CoV-2 XBB.1.5 mRNA booster vaccination elicits limited mucosal immunity.Sci Transl Med. 2024 Oct 23;16(770):eadp8920. doi: 10.1126/scitranslmed.adp8920. Epub 2024 Oct 23. Sci Transl Med. 2024. PMID: 39441905 Free PMC article.
-
Effects of Rotavirus NSP4 on the Immune Response and Protection of Rotavirus-Norovirus Recombinant Subunit Vaccines in Different Immune Pathways.Vaccines (Basel). 2024 Sep 8;12(9):1025. doi: 10.3390/vaccines12091025. Vaccines (Basel). 2024. PMID: 39340055 Free PMC article.
References
-
- Siddle K. J., Krasilnikova L. A., Moreno G. K., Schaffner S. F., Vostok J., Fitzgerald N. A., Lemieux J. E., Barkas N., Loreth C., Specht I., Tomkins-Tinch C. H., Paull J. S., Schaeffer B., Taylor B. P., Loftness B., Johnson H., Schubert P. L., Shephard H. M., Doucette M., Fink T., Lang A. S., Baez S., Beauchamp J., Hennigan S., Buzby E., Ash S., Brown J., Clancy S., Cofsky S., Gagne L., Hall J., Harrington R., Gionet G. L., DeRuff K. C., Vodzak M. E., Adams G. C., Dobbins S. T., Slack S. D., Reilly S. K., Anderson L. M., Cipicchio M. C., DeFelice M. T., Grimsby J. L., Anderson S. E., Blumenstiel B. S., Meldrim J. C., Rooke H. M., Vicente G., Smith N. L., Messer K. S., Reagan F. L., Mandese Z. M., Lee M. D., Ray M. C., Fisher M. E., Ulcena M. A., Nolet C. M., English S. E., Larkin K. L., Vernest K., Chaluvadi S., Arvidson D., Melchiono M., Covell T., Harik V., Brock-Fisher T., Dunn M., Kearns A., Hanage W. P., Bernard C., Philippakis A., Lennon N. J., Gabriel S. B., Gallagher G. R., Smole S., Madoff L. C., Brown C. M., Park D. J., MacInnis B. L., Sabeti P. C., Transmission from vaccinated individuals in a large SARS-CoV-2 Delta variant outbreak. Cell 185, 485–492.e10 (2022). 10.1016/j.cell.2021.12.027 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- U54 CA260582/CA/NCI NIH HHS/United States
- R01 NS103212/NS/NINDS NIH HHS/United States
- R01 AI157852/AI/NIAID NIH HHS/United States
- R01 AI147394/AI/NIAID NIH HHS/United States
- RF1 NS122174/NS/NINDS NIH HHS/United States
- P01 AI158571/AI/NIAID NIH HHS/United States
- R01 NS122174/NS/NINDS NIH HHS/United States
- R01 AI150473/AI/NIAID NIH HHS/United States
- R01 AI057459/AI/NIAID NIH HHS/United States
- R01 AG047156/AG/NIA NIH HHS/United States
- R21 AI147903/AI/NIAID NIH HHS/United States
- R01 AG069264/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous


