Osteocalcin Alleviates Lipopolysaccharide-Induced Acute Inflammation via Activation of GPR37 in Macrophages
- PMID: 35625743
- PMCID: PMC9138386
- DOI: 10.3390/biomedicines10051006
Osteocalcin Alleviates Lipopolysaccharide-Induced Acute Inflammation via Activation of GPR37 in Macrophages
Abstract
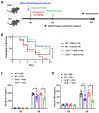
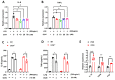
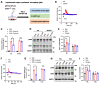
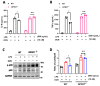
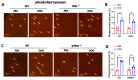
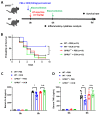
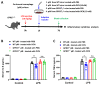
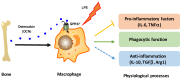
The G protein-coupled receptor 37 (GPR37) has been reported to be expressed in macrophages and the activation of GPR37 by its ligand/agonist, and it can regulate macrophage-associated functions and inflammatory responses. Since our previous work identified that osteocalcin (OCN) acts as an endogenous ligand for GPR37 and can elicit various intracellular signals by interacting with GPR37, we thus hypothesized that OCN may also play a functional role in macrophage through the activation of GPR37. To verify the hypothesis, we conducted a series of in vivo and in vitro studies in lipopolysaccharide (LPS)-challenged mice and primary cultured macrophages. Our results reveal that the OCN gene deletion (OCN-/-) and wild type (WT) mice showed comparable death rates and inflammatory cytokines productions in response to a lethal dose of LPS exposure. However, the detrimental effects caused by LPS were significantly ameliorated by exogenous OCN treatments in both WT and OCN-/- mice. Notably, the protective effects of OCN were absent in GPR37-/- mice. In coordination with the in vivo results, our in vitro studies further illustrated that OCN triggered intracellular responses via GPR37 in peritoneal macrophages by regulating the release of inflammatory factors and macrophage phagocytic function. Finally, we exhibited that the adoptive transfer of OCN-treated macrophages from WT mice significantly inhibits the release of pro-inflammatory cytokines in GPR37-/- mice exposed to LPS. Taken together, these findings suggest a protective role of OCN against LPS-caused acute inflammation, by the activation of GPR37 in macrophages, and provide a potential application of the activation of the OCN/GPR37 regulatory axis as a therapeutic strategy for inflammatory diseases.
Keywords: GPR37; acute inflammation; lipopolysaccharide; macrophage; osteocalcin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Inflammation and Infection in Pain and the Role of GPR37.Int J Mol Sci. 2022 Nov 20;23(22):14426. doi: 10.3390/ijms232214426. Int J Mol Sci. 2022. PMID: 36430912 Free PMC article. Review.
-
GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain.J Clin Invest. 2018 Aug 1;128(8):3568-3582. doi: 10.1172/JCI99888. Epub 2018 Jul 16. J Clin Invest. 2018. PMID: 30010619 Free PMC article.
-
Osteocalcin attenuates oligodendrocyte differentiation and myelination via GPR37 signaling in the mouse brain.Sci Adv. 2021 Oct 22;7(43):eabi5811. doi: 10.1126/sciadv.abi5811. Epub 2021 Oct 22. Sci Adv. 2021. PMID: 34678058 Free PMC article.
-
Activation of GPR37 in macrophages confers protection against infection-induced sepsis and pain-like behaviour in mice.Nat Commun. 2021 Mar 17;12(1):1704. doi: 10.1038/s41467-021-21940-8. Nat Commun. 2021. PMID: 33731716 Free PMC article.
-
Emerging roles of the G-protein-coupled receptor 37 in neurological diseases and pain.Neuroscience. 2024 Nov 1;559:199-208. doi: 10.1016/j.neuroscience.2024.08.032. Epub 2024 Sep 6. Neuroscience. 2024. PMID: 39244010 Review.
Cited by
-
An inflammatory liquid fingerprint predicting tumor recurrence after liver transplantation for hepatocellular carcinoma.MedComm (2020). 2024 Aug 26;5(9):e678. doi: 10.1002/mco2.678. eCollection 2024 Sep. MedComm (2020). 2024. PMID: 39188937 Free PMC article.
-
Orphan G Protein-Coupled Receptor GPR37 as an Emerging Therapeutic Target.ACS Chem Neurosci. 2023 Sep 20;14(18):3318-3334. doi: 10.1021/acschemneuro.3c00479. Epub 2023 Sep 7. ACS Chem Neurosci. 2023. PMID: 37676000 Free PMC article. Review.
-
Inflammation and Infection in Pain and the Role of GPR37.Int J Mol Sci. 2022 Nov 20;23(22):14426. doi: 10.3390/ijms232214426. Int J Mol Sci. 2022. PMID: 36430912 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources


