Diversity and ecology of Radiolaria in modern oceans
- PMID: 35412019
- PMCID: PMC9322464
- DOI: 10.1111/1462-2920.16004
Diversity and ecology of Radiolaria in modern oceans
Abstract
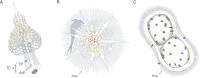
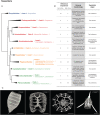
Among the many inhabitants of planktonic communities, several lineages have biomineralized intricate skeletons. These have existed for millions of years and include the Radiolaria, a group of marine protists, many of which bear delicate mineral skeletons of different natures. Radiolaria are well known for their paleontological signatures, but little is known about the ecology of modern assemblages. They are found from polar to tropical regions, in the sunlit layers of the ocean down to the deep and cold bathypelagic. They are closely involved in the biogeochemical cycles of silica, carbon and strontium sulfate, carrying important amounts of such elements to the deep ocean. However, relatively little is known on the actual extent of genetic diversity or biogeographic patterns. The rapid emergence and acceptance of molecular approaches have nevertheless led to major advances in our understanding of diversity within and evolutionary relationships between major radiolarian groups. Here, we review the state of knowledge relating to the classification, diversity and ecology of extant radiolarian orders, highlighting the substantial gaps in our understanding of the extent of their contribution to marine biodiversity and their role in marine food webs.
© 2022 The Author. Environmental Microbiology published by Society for Applied Microbiology and John Wiley & Sons Ltd.
Figures





Similar articles
-
Ocean plankton. Eukaryotic plankton diversity in the sunlit ocean.Science. 2015 May 22;348(6237):1261605. doi: 10.1126/science.1261605. Science. 2015. PMID: 25999516
-
Hydroids (Cnidaria, Hydrozoa) from Mauritanian Coral Mounds.Zootaxa. 2020 Nov 16;4878(3):zootaxa.4878.3.2. doi: 10.11646/zootaxa.4878.3.2. Zootaxa. 2020. PMID: 33311142
-
Diversity and biogeochemical function of planktonic fungi in the ocean.Prog Mol Subcell Biol. 2012;53:71-88. doi: 10.1007/978-3-642-23342-5_4. Prog Mol Subcell Biol. 2012. PMID: 22222827
-
Marine protist associations and environmental impacts across trophic levels in the twilight zone and below.Curr Opin Microbiol. 2016 Jun;31:169-175. doi: 10.1016/j.mib.2016.04.001. Epub 2016 Apr 17. Curr Opin Microbiol. 2016. PMID: 27092409 Review.
-
Protist diversity and function in the dark ocean - Challenging the paradigms of deep-sea ecology with special emphasis on foraminiferans and naked protists.Eur J Protistol. 2020 Aug;75:125721. doi: 10.1016/j.ejop.2020.125721. Epub 2020 Jun 4. Eur J Protistol. 2020. PMID: 32575029 Review.
Cited by
-
Distinct Communities of Bacteria and Unicellular Eukaryotes in the Different Water Masses of Cretan Passage Water Column (Eastern Mediterranean Sea).Curr Microbiol. 2024 Sep 28;81(11):381. doi: 10.1007/s00284-024-03906-3. Curr Microbiol. 2024. PMID: 39340560
-
Marine microfossils: Tiny archives of ocean changes through deep time.AIMS Microbiol. 2024 Aug 8;10(3):644-673. doi: 10.3934/microbiol.2024030. eCollection 2024. AIMS Microbiol. 2024. PMID: 39219758 Free PMC article. Review.
-
Global census of the significance of giant mesopelagic protists to the marine carbon and silicon cycles.Nat Commun. 2024 Apr 29;15(1):3341. doi: 10.1038/s41467-024-47651-4. Nat Commun. 2024. PMID: 38684684 Free PMC article.
-
Role of Syndiniales parasites in depth-specific networks and carbon flux in the oligotrophic ocean.ISME Commun. 2024 Jan 23;4(1):ycae014. doi: 10.1093/ismeco/ycae014. eCollection 2024 Jan. ISME Commun. 2024. PMID: 38419659 Free PMC article.
-
Subtropical coastal microbiome variations due to massive river runoff after a cyclonic event.Environ Microbiome. 2024 Jan 30;19(1):10. doi: 10.1186/s40793-024-00554-9. Environ Microbiome. 2024. PMID: 38291506 Free PMC article.
References
-
- Anderson, O.R. (1977) Cytoplasmic fine structure of nassellarian Radiolaria. Mar Micropaleontol 2: 251–264.
-
- Anderson, O.R. (1978) Light and electron microscopic observations of feeding behavior, nutrition, and reproduction in laboratory cultures of Thalassicolla nucleata. Tissue Cell 10: 401–412. - PubMed
-
- Anderson, O.R. (1983) Radiolaria: New York: Springer‐Verlag.
-
- Anderson, O.R. (2012) Living together in the plankton: a survey of marine protist symbioses. Acta Protozool 52: 1–10.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources


