The Rotavirus Vaccine Story: From Discovery to the Eventual Control of Rotavirus Disease
- PMID: 34590142
- PMCID: PMC8482027
- DOI: 10.1093/infdis/jiaa598
The Rotavirus Vaccine Story: From Discovery to the Eventual Control of Rotavirus Disease
Abstract
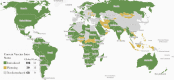
Worldwide, rotavirus is the leading pathogen causing severe diarrhea in children and a major cause of under 5 years mortality. In 1998, the first rotavirus vaccine, RotaShield, was licensed in the United States but a rare adverse event, intussusception, led to its withdrawal. Seven years passed before the next generation of vaccines became available, Rotarix (GSK) and Rotateq (Merck), and 11 years later, 2 additional vaccines from India, Rotavac (Bharat) and Rotasiil (Serum Institute), were recommended by World Health Organization for all children. Today, these vaccines are used in more than 100 countries and have contributed to marked decreases in hospitalizations and deaths from diarrhea. However, these live oral vaccines are less effective in low-income countries with high under 5 years mortality for reasons that are not understood. Efforts to develop new vaccines that avoid the oral route are in progress and will likely be needed to ultimately control rotavirus disease.
Keywords: child survival; childhood diarrhea; rotavirus; vaccine.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

Similar articles
-
Vaccines for preventing rotavirus diarrhoea: vaccines in use.Cochrane Database Syst Rev. 2021 Nov 17;11(11):CD008521. doi: 10.1002/14651858.CD008521.pub6. Cochrane Database Syst Rev. 2021. PMID: 34788488 Free PMC article. Review.
-
Rotavirus vaccines: targeting the developing world.J Infect Dis. 2005 Sep 1;192 Suppl 1:S160-6. doi: 10.1086/431504. J Infect Dis. 2005. PMID: 16088799
-
[New rotavirus vaccines: a reality at last].Rev Chilena Infectol. 2005 Dec;22(4):345-54. doi: 10.4067/s0716-10182005000600007. Epub 2005 Dec 5. Rev Chilena Infectol. 2005. PMID: 16341356 Review. Spanish.
-
Global Experience With Rotavirus Vaccines.J Infect Dis. 2021 Dec 20;224(12 Suppl 2):S792-S800. doi: 10.1093/infdis/jiab399. J Infect Dis. 2021. PMID: 34374426 Free PMC article.
-
Product review of the rotavirus vaccines ROTASIIL, ROTAVAC, and Rotavin-M1.Hum Vaccin Immunother. 2021 Apr 3;17(4):1223-1234. doi: 10.1080/21645515.2020.1804245. Epub 2020 Oct 29. Hum Vaccin Immunother. 2021. PMID: 33121329 Free PMC article. Review.
Cited by
-
Factors associated with antibiotic use in children hospitalized for acute viral gastroenteritis and the relation to rotavirus vaccination.Hum Vaccin Immunother. 2024 Dec 31;20(1):2396707. doi: 10.1080/21645515.2024.2396707. Epub 2024 Sep 9. Hum Vaccin Immunother. 2024. PMID: 39248509 Free PMC article.
-
Effect of rotavirus vaccination on the burden of rotavirus disease and associated antibiotic use in India: A dynamic agent-based simulation analysis.Vaccine. 2024 Sep 17;42(22):126211. doi: 10.1016/j.vaccine.2024.126211. Epub 2024 Aug 12. Vaccine. 2024. PMID: 39137492 Free PMC article.
-
Navigating Viral Gastroenteritis: Epidemiological Trends, Pathogen Analysis, and Histopathological Findings.Cureus. 2024 May 27;16(5):e61197. doi: 10.7759/cureus.61197. eCollection 2024 May. Cureus. 2024. PMID: 38939260 Free PMC article.
-
Reverse Genetics of Murine Rotavirus: A Comparative Analysis of the Wild-Type and Cell-Culture-Adapted Murine Rotavirus VP4 in Replication and Virulence in Neonatal Mice.Viruses. 2024 May 12;16(5):767. doi: 10.3390/v16050767. Viruses. 2024. PMID: 38793648 Free PMC article.
-
Correlates of immune protection against human rotaviruses: natural infection and vaccination.Arch Virol. 2024 Mar 8;169(3):72. doi: 10.1007/s00705-024-05975-y. Arch Virol. 2024. PMID: 38459213 Review.
References
-
- Bishop RF, Davidson GP, Holmes IH, Ruck BJ. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet 1973; 2:1281–3. - PubMed
-
- Institute of Medicine. The prospects of immunizing against rotavirus. New vaccine development: diseases of importance in developing countries. Washington, DC: National Academy Press, 1986.
-
- Estes MK, Kapikian AZ. Rotaviruses. In: Knipe DM, Howley PM, eds. Field’s virology. 5th ed. Philadelphia, PA: Lippincott, Williams, and Williams, 2007:1917–58.
-
- Kapikian AZ, Hoshino Y, Chanock RM, Pérez-Schael I. Efficacy of a quadrivalent rhesus rotavirus-based human rotavirus vaccine aimed at preventing severe rotavirus diarrhea in infants and young children. J Infect Dis 1996; 174(Suppl 1):S65–72. - PubMed
-
- Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1999; 48:1–20. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical


