Wnt/β-catenin signaling pathway in uterine leiomyoma: role in tumor biology and targeting opportunities
- PMID: 33999334
- PMCID: PMC9235413
- DOI: 10.1007/s11010-021-04174-6
Wnt/β-catenin signaling pathway in uterine leiomyoma: role in tumor biology and targeting opportunities
Abstract
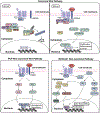
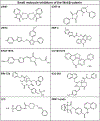
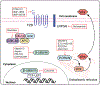
Uterine leiomyoma is the most common tumor of the female reproductive system and originates from a single transformed myometrial smooth muscle cell. Despite the immense medical, psychosocial, and financial impact, the exact underlying mechanisms of leiomyoma pathobiology are poorly understood. Alterations of signaling pathways are thought to be instrumental in leiomyoma biology. Wnt/β-catenin pathway appears to be involved in several aspects of the genesis of leiomyomas. For example, Wnt5b is overexpressed in leiomyoma, and the Wnt/β-catenin pathway appears to mediate the role of MED12 mutations, the most common mutations in leiomyoma, in tumorigenesis. Moreover, Wnt/β-catenin pathway plays a paracrine role where estrogen/progesterone treatment of mature myometrial or leiomyoma cells leads to increased expression of Wnt11 and Wnt16, which induces proliferation of leiomyoma stem cells and tumor growth. Constitutive activation of β-catenin leads to myometrial hyperplasia and leiomyoma-like lesions in animal models. Wnt/β-catenin signaling is also closely involved in mechanotransduction and extracellular matrix regulation and relevant alterations in leiomyoma, and crosstalk is noted between Wnt/β-catenin signaling and other pathways known to regulate leiomyoma development and growth such as estrogen, progesterone, TGFβ, PI3K/Akt/mTOR, Ras/Raf/MEK/ERK, IGF, Hippo, and Notch signaling. Finally, evidence suggests that inhibition of the canonical Wnt pathway using β-catenin inhibitors inhibits leiomyoma cell proliferation. Understanding the molecular mechanisms of leiomyoma development is essential for effective treatment. The specific Wnt/β-catenin pathway molecules discussed in this review constitute compelling candidates for therapeutic targeting.
Keywords: Leiomyoma; Pathobiology; Signaling pathway; Uterine fibroid; Wnt/β-catenin pathway.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Figures
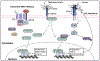
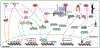
Similar articles
-
Paracrine activation of WNT/β-catenin pathway in uterine leiomyoma stem cells promotes tumor growth.Proc Natl Acad Sci U S A. 2013 Oct 15;110(42):17053-8. doi: 10.1073/pnas.1313650110. Epub 2013 Sep 30. Proc Natl Acad Sci U S A. 2013. PMID: 24082114 Free PMC article. Clinical Trial.
-
Inhibition of tumor cell proliferation in human uterine leiomyomas by vitamin D via Wnt/β-catenin pathway.Fertil Steril. 2019 Feb;111(2):397-407. doi: 10.1016/j.fertnstert.2018.10.008. Epub 2018 Nov 17. Fertil Steril. 2019. PMID: 30458994
-
Guizhi Fuling Capsule inhibits uterine fibroids growth by modulating Med12-mediated Wnt/β-Catenin signaling pathway.J Ethnopharmacol. 2022 May 23;290:115115. doi: 10.1016/j.jep.2022.115115. Epub 2022 Feb 16. J Ethnopharmacol. 2022. PMID: 35181487
-
Uterine Leiomyoma Stem Cells: Linking Progesterone to Growth.Semin Reprod Med. 2015 Sep;33(5):357-65. doi: 10.1055/s-0035-1558451. Epub 2015 Aug 6. Semin Reprod Med. 2015. PMID: 26251118 Review.
-
Epidemiological and genetic clues for molecular mechanisms involved in uterine leiomyoma development and growth.Hum Reprod Update. 2015 Sep-Oct;21(5):593-615. doi: 10.1093/humupd/dmv030. Epub 2015 Jul 3. Hum Reprod Update. 2015. PMID: 26141720 Free PMC article. Review.
Cited by
-
Estrogen receptor alpha mediated repression of PRICKLE1 destabilizes REST and promotes uterine fibroid pathogenesis.bioRxiv [Preprint]. 2024 Sep 9:2024.09.09.612036. doi: 10.1101/2024.09.09.612036. bioRxiv. 2024. PMID: 39314474 Free PMC article. Preprint.
-
Hypoxia in uterine fibroids: role in pathobiology and therapeutic opportunities.Oxygen (Basel). 2024 Jun;4(2):236-252. doi: 10.3390/oxygen4020013. Epub 2024 May 28. Oxygen (Basel). 2024. PMID: 38957794 Free PMC article.
-
Genetic Mechanisms Driving Uterine Leiomyoma Pathobiology, Epidemiology, and Treatment.Genes (Basel). 2024 Apr 27;15(5):558. doi: 10.3390/genes15050558. Genes (Basel). 2024. PMID: 38790186 Free PMC article. Review.
-
Targeting fibrotic signaling pathways by EGCG as a therapeutic strategy for uterine fibroids.Sci Rep. 2023 May 25;13(1):8492. doi: 10.1038/s41598-023-35212-6. Sci Rep. 2023. PMID: 37231028 Free PMC article.
-
EZH2 activates Wnt/β-catenin signaling in human uterine fibroids, which is inhibited by the natural compound methyl jasmonate.F S Sci. 2023 Aug;4(3):239-256. doi: 10.1016/j.xfss.2023.05.003. Epub 2023 May 12. F S Sci. 2023. PMID: 37182601 Free PMC article.
References
-
- Gupta S, Jose J, Manyonda I. Clinical presentation of fibroids. Best practice & research Clinical obstetrics & gynaecology. 2008;22(4):615–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous


