High endothelial venules (HEVs) in immunity, inflammation and cancer
- PMID: 33956259
- PMCID: PMC8487881
- DOI: 10.1007/s10456-021-09792-8
High endothelial venules (HEVs) in immunity, inflammation and cancer
Abstract
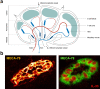
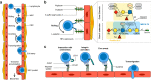
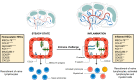
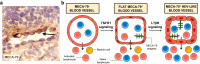
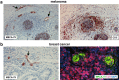
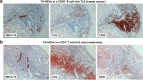
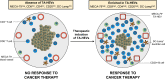
High endothelial venules (HEVs) are specialized blood vessels mediating lymphocyte trafficking to lymph nodes (LNs) and other secondary lymphoid organs. By supporting high levels of lymphocyte extravasation from the blood, HEVs play an essential role in lymphocyte recirculation and immune surveillance for foreign invaders (bacterial and viral infections) and alterations in the body's own cells (neoantigens in cancer). The HEV network expands during inflammation in immune-stimulated LNs and is profoundly remodeled in metastatic and tumor-draining LNs. HEV-like blood vessels expressing high levels of the HEV-specific sulfated MECA-79 antigens are induced in non-lymphoid tissues at sites of chronic inflammation in many human inflammatory and allergic diseases, including rheumatoid arthritis, Crohn's disease, allergic rhinitis and asthma. Such vessels are believed to contribute to the amplification and maintenance of chronic inflammation. MECA-79+ tumor-associated HEVs (TA-HEVs) are frequently found in human tumors in CD3+ T cell-rich areas or CD20+ B-cell rich tertiary lymphoid structures (TLSs). TA-HEVs have been proposed to play important roles in lymphocyte entry into tumors, a process essential for successful antitumor immunity and lymphocyte-mediated cancer immunotherapy with immune checkpoint inhibitors, vaccines or adoptive T cell therapy. In this review, we highlight the phenotype and function of HEVs in homeostatic, inflamed and tumor-draining lymph nodes, and those of HEV-like blood vessels in chronic inflammatory diseases. Furthermore, we discuss the role and regulation of TA-HEVs in human cancer and mouse tumor models.
Keywords: Cancer immunology; Chronic inflammatory diseases; High endothelial venules (HEVs); Lymphocyte trafficking; Tertiary lymphoid structures; Tumor blood vessels.
© 2021. The Author(s).
Figures
Similar articles
-
High Endothelial Venules and Other Blood Vessels: Critical Regulators of Lymphoid Organ Development and Function.Front Immunol. 2017 Feb 3;8:45. doi: 10.3389/fimmu.2017.00045. eCollection 2017. Front Immunol. 2017. PMID: 28217126 Free PMC article. Review.
-
High endothelial venules in cancer: Regulation, function, and therapeutic implication.Cancer Cell. 2023 Mar 13;41(3):527-545. doi: 10.1016/j.ccell.2023.02.002. Epub 2023 Feb 23. Cancer Cell. 2023. PMID: 36827979 Review.
-
Defining High Endothelial Venules and Tertiary Lymphoid Structures in Cancer.Methods Mol Biol. 2018;1845:99-118. doi: 10.1007/978-1-4939-8709-2_7. Methods Mol Biol. 2018. PMID: 30141010
-
High endothelial venules in intracranial germinomas: Implications for lymphocytes infiltration.Cancer Med. 2023 Mar;12(5):5450-5460. doi: 10.1002/cam4.5367. Epub 2022 Oct 19. Cancer Med. 2023. PMID: 36259639 Free PMC article.
-
High Endothelial Venules: A Vascular Perspective on Tertiary Lymphoid Structures in Cancer.Front Immunol. 2021 Aug 17;12:736670. doi: 10.3389/fimmu.2021.736670. eCollection 2021. Front Immunol. 2021. PMID: 34484246 Free PMC article. Review.
Cited by
-
Methods for Selective Gene Expression Profiling in Single Tertiary Lymphoid Structure Using Laser Capture Microdissection.Methods Mol Biol. 2025;2864:107-126. doi: 10.1007/978-1-0716-4184-2_6. Methods Mol Biol. 2025. PMID: 39527219
-
Tertiary Lymphoid Structures in Central Nervous System Disorders.Methods Mol Biol. 2025;2864:21-42. doi: 10.1007/978-1-0716-4184-2_2. Methods Mol Biol. 2025. PMID: 39527215 Review.
-
Epithelioid pleural mesothelioma successfully treated with perioperative immunotherapy: a case report.Gen Thorac Cardiovasc Surg Cases. 2024 Jun 11;3(1):31. doi: 10.1186/s44215-024-00157-3. Gen Thorac Cardiovasc Surg Cases. 2024. PMID: 39517020 Free PMC article.
-
Clarification of a unique mucosal vaccination route for improved systemic and mucosal immune response in broiler.Heliyon. 2024 Oct 19;10(20):e39621. doi: 10.1016/j.heliyon.2024.e39621. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39497952 Free PMC article.
-
Tertiary Lymphoid Structures in Microorganism-Related Cancer.Cancers (Basel). 2024 Oct 12;16(20):3464. doi: 10.3390/cancers16203464. Cancers (Basel). 2024. PMID: 39456558 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


