Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study
- PMID: 33901420
- PMCID: PMC8064669
- DOI: 10.1016/S0140-6736(21)00677-2
Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study
Abstract
Background: The BNT162b2 mRNA (Pfizer-BioNTech) and ChAdOx1 nCoV-19 (Oxford-AstraZeneca) COVID-19 vaccines have shown high efficacy against disease in phase 3 clinical trials and are now being used in national vaccination programmes in the UK and several other countries. Studying the real-world effects of these vaccines is an urgent requirement. The aim of our study was to investigate the association between the mass roll-out of the first doses of these COVID-19 vaccines and hospital admissions for COVID-19.
Methods: We did a prospective cohort study using the Early Pandemic Evaluation and Enhanced Surveillance of COVID-19-EAVE II-database comprising linked vaccination, primary care, real-time reverse transcription-PCR testing, and hospital admission patient records for 5·4 million people in Scotland (about 99% of the population) registered at 940 general practices. Individuals who had previously tested positive were excluded from the analysis. A time-dependent Cox model and Poisson regression models with inverse propensity weights were fitted to estimate effectiveness against COVID-19 hospital admission (defined as 1-adjusted rate ratio) following the first dose of vaccine.
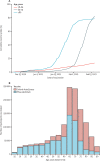
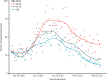
Findings: Between Dec 8, 2020, and Feb 22, 2021, a total of 1 331 993 people were vaccinated over the study period. The mean age of those vaccinated was 65·0 years (SD 16·2). The first dose of the BNT162b2 mRNA vaccine was associated with a vaccine effect of 91% (95% CI 85-94) for reduced COVID-19 hospital admission at 28-34 days post-vaccination. Vaccine effect at the same time interval for the ChAdOx1 vaccine was 88% (95% CI 75-94). Results of combined vaccine effects against hospital admission due to COVID-19 were similar when restricting the analysis to those aged 80 years and older (83%, 95% CI 72-89 at 28-34 days post-vaccination).
Interpretation: Mass roll-out of the first doses of the BNT162b2 mRNA and ChAdOx1 vaccines was associated with substantial reductions in the risk of hospital admission due to COVID-19 in Scotland. There remains the possibility that some of the observed effects might have been due to residual confounding.
Funding: UK Research and Innovation (Medical Research Council), Research and Innovation Industrial Strategy Challenge Fund, Health Data Research UK.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests AS and JMcM are members of the Scottish Government chief medical officer's COVID-19 Advisory Group. JMcM is a member of the New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG) and AS is a member of the NERVTAG Risk Stratification Subgroup. JMcM is a member of the Scientific Advisory Group on Emergencies (SAGE) and chairs the COVID Scottish National Incident Management Team and the Scientific Committee of the European Centre for Disease Prevention and Control-funded and WHO-funded IMOVE-COVID-19 group. CRS declares funding from the Medical Research Council, the National Institute of Health Research, Chief Scientist Office, and New Zealand Ministry for Business, Innovation and Employment and Health Research Council during the conduct of this study. SVK is co-chair of the Scottish Government's Expert Reference Group on COVID-19 and ethnicity; is a member of the SAGE subgroup on ethnicity; and acknowledges funding from a National Health Service Research Scotland Senior Clinical Fellowship, the Medical Research Council, and Chief Scientist Office. CR is a member of the Scottish Government chief medical officer's COVID-19 Advisory Group, Scientific Pandemic Influenza Group on Modelling, and Medicines and Healthcare products Regulatory Agency Vaccine Benefit and Risk Working Group. DB reports employment by Queen's University Belfast, Public Health Agency, and a secondment to Northern Ireland's Department of Health. SdL reports that he is the director of the Oxford-Royal College of General Practitioners Research and Surveillance Centre and that its principal funder is Public Health England. SdL has received funding through the University of Oxford for studies from AstraZeneca, Daiichi Sankyo, Eli Lilly, Sanofi, GSK, Merck Sharp & Dohme, Seqirus, and Takeda; and has been a member of advisory boards for influenza for Seqirus and Sanofi. FDRH reports that the University of Oxford received research funding from Her Majesty's Government for the ChadOx1 vaccine trial programme and entered a partnership with AstraZeneca for the manufacture and distribution of the vaccine. FDRH was not involved in these relationships. All other authors report no competing interests.
Figures

Comment in
-
Hospital admissions due to COVID-19 in Scotland after one dose of vaccine.Lancet. 2021 May 1;397(10285):1601-1603. doi: 10.1016/S0140-6736(21)00765-0. Epub 2021 Apr 23. Lancet. 2021. PMID: 33901421 Free PMC article. No abstract available.
-
Characteristics and risk of COVID-19-related death in fully vaccinated people in Scotland.Lancet. 2021 Nov 13;398(10313):1799-1800. doi: 10.1016/S0140-6736(21)02316-3. Epub 2021 Oct 28. Lancet. 2021. PMID: 34756204 Free PMC article. No abstract available.
Similar articles
-
COVID-19 hospital admissions and deaths after BNT162b2 and ChAdOx1 nCoV-19 vaccinations in 2·57 million people in Scotland (EAVE II): a prospective cohort study.Lancet Respir Med. 2021 Dec;9(12):1439-1449. doi: 10.1016/S2213-2600(21)00380-5. Epub 2021 Sep 29. Lancet Respir Med. 2021. PMID: 34599903 Free PMC article.
-
Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study.BMJ. 2021 May 13;373:n1088. doi: 10.1136/bmj.n1088. BMJ. 2021. PMID: 33985964 Free PMC article.
-
Two-dose ChAdOx1 nCoV-19 vaccine protection against COVID-19 hospital admissions and deaths over time: a retrospective, population-based cohort study in Scotland and Brazil.Lancet. 2022 Jan 1;399(10319):25-35. doi: 10.1016/S0140-6736(21)02754-9. Epub 2021 Dec 20. Lancet. 2022. PMID: 34942103 Free PMC article.
-
Clinical characteristics, radiological features and prognostic factors of transverse myelitis following COVID-19 vaccination: A systematic review.Mult Scler Relat Disord. 2022 Oct;66:104032. doi: 10.1016/j.msard.2022.104032. Epub 2022 Jul 6. Mult Scler Relat Disord. 2022. PMID: 35858499 Free PMC article. Review.
-
Learning from the COVID-19 pandemic: A systematic review of mathematical vaccine prioritization models.Infect Dis Model. 2024 May 15;9(4):1057-1080. doi: 10.1016/j.idm.2024.05.005. eCollection 2024 Dec. Infect Dis Model. 2024. PMID: 38988830 Free PMC article. Review.
Cited by
-
Impact of SARS-CoV-2 infection and vaccination on cesarean section outcomes: a retrospective analysis.Ann Saudi Med. 2024 Sep-Oct;44(5):306-318. doi: 10.5144/0256-4947.2024.306. Epub 2024 Oct 3. Ann Saudi Med. 2024. PMID: 39368119 Free PMC article.
-
Asymptomatic infection and disappearance of clinical symptoms of COVID-19 infectors in China 2022-2023: a cross-sectional study.Sci Rep. 2024 Aug 6;14(1):18232. doi: 10.1038/s41598-024-68162-8. Sci Rep. 2024. PMID: 39107338 Free PMC article.
-
Healthcare as a driver, reservoir and amplifier of antimicrobial resistance: opportunities for interventions.Nat Rev Microbiol. 2024 Oct;22(10):636-649. doi: 10.1038/s41579-024-01076-4. Epub 2024 Jul 24. Nat Rev Microbiol. 2024. PMID: 39048837 Review.
-
Urgent support mechanism: saving millions of COVID-19 vaccines from expiry in Africa.BMJ Glob Health. 2024 Jun 6;9(6):e015181. doi: 10.1136/bmjgh-2024-015181. BMJ Glob Health. 2024. PMID: 38844381 Free PMC article.
-
Producing Plasmid DNA Template for Clinical Grade RNA Vaccine Manufacture.Methods Mol Biol. 2024;2786:303-319. doi: 10.1007/978-1-0716-3770-8_14. Methods Mol Biol. 2024. PMID: 38814401
References
-
- UK Government Coronavirus in the UK. 2021. https://coronavirus.data.gov.uk/
-
- UK Government Joint Committee on Vaccination and Immunisation: advice on priority groups for COVID-19 vaccination, 30 December 2020. 2021. https://www.gov.uk/government/publications/priority-groups-for-coronavir...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials


