Expression and Characterization of a Thermostable Carrageenase From an Antarctic Polaribacter sp. NJDZ03 Strain
- PMID: 33776960
- PMCID: PMC7994522
- DOI: 10.3389/fmicb.2021.631039
Expression and Characterization of a Thermostable Carrageenase From an Antarctic Polaribacter sp. NJDZ03 Strain
Abstract
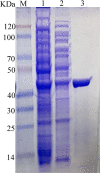
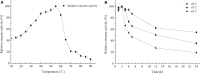
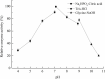
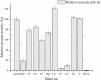
The complete genome of Polaribacter sp. NJDZ03, which was isolated from the surface of Antarctic macroalgae, was analyzed by next-generation sequencing, and a putative carrageenase gene Car3206 was obtained. Car3206 was cloned and expressed in Escherichia coli BL21(DE3). After purification by Ni-NTA chromatography, the recombinant Car3206 protein was characterized and the antioxidant activity of the degraded product was investigated. The results showed that the recombinant plasmid pet-30a-car3206 was highly efficiently expressed in E. coli BL21(DE3). The purified recombinant Car3206 showed a single band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, with an apparent molecular weight of 45 kDa. The optimum temperature of the recombinant Car3206 was 55°C, and it maintain 60-94% of its initial activity for 4-12 h at 55°C. It also kept almost 70% of the initial activity at 30°C, and more than 40% of the initial activity at 10°C. These results show that recombinant Car3206 had good low temperature resistance and thermal stability properties. The optimum pH of recombinant Car3206 was 7.0. Car3206 was activated by Na+, K+, and Ca2+, but was significantly inhibited by Cu2+ and Cr2+. Thin-layer chromatographic analysis indicated that Car3206 degraded carrageenan generating disaccharides as the only products. The antioxidant capacity of the degraded disaccharides in vitro was investigated and the results showed that different concentrations of the disaccharides had similar scavenging effects as vitamin C on , •OH, and DPPH•. To our knowledge, this is the first report about details of the biochemical characteristics of a carrageenase isolated from an Antarctic Polaribacter strain. The unique characteristics of Car3206, including its low temperature resistance, thermal stability, and product unity, suggest that this enzyme may be an interesting candidate for industrial processes.
Keywords: antioxidant capacity; carrageenase; enzymatic characterization; enzymatic hydrolysis; gene expression.
Copyright © 2021 Gui, Gu, Fu, Zhang, Zhang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Biochemical characterization and elucidation of the action mode of a GH16 family κ-carrageenase for efficient preparation of carrageenan oligosaccharides.World J Microbiol Biotechnol. 2023 Jun 7;39(8):222. doi: 10.1007/s11274-023-03668-3. World J Microbiol Biotechnol. 2023. PMID: 37285044
-
[Expression and characterization of the agarase gene aga3311 from an Antarctic bacterium].Wei Sheng Wu Xue Bao. 2016 Sep;56(9):1468-76. Wei Sheng Wu Xue Bao. 2016. PMID: 29738219 Chinese.
-
Purification and characterization of a thermostable λ-carrageenase from a hot spring bacterium, Bacillus sp.Biotechnol Lett. 2014 Aug;36(8):1669-74. doi: 10.1007/s10529-014-1520-7. Epub 2014 Apr 16. Biotechnol Lett. 2014. PMID: 24737075
-
Biochemical Characterization of a Carrageenase, Car1383, Derived From Associated Bacteria of Antarctic Macroalgae.Front Microbiol. 2022 Mar 31;13:851182. doi: 10.3389/fmicb.2022.851182. eCollection 2022. Front Microbiol. 2022. PMID: 35432236 Free PMC article.
-
Purification and characterization of chitinase from Paenibacillus sp. .Biotechnol Appl Biochem. 2021 Feb;68(1):30-40. doi: 10.1002/bab.1889. Epub 2020 Feb 19. Biotechnol Appl Biochem. 2021. PMID: 31957084 Review.
Cited by
-
Expanding the application range of the κ‑carrageenase OUC-FaKC16A when preparing oligosaccharides from κ-carrageenan and furcellaran.Mar Life Sci Technol. 2023 Jul 12;5(3):387-399. doi: 10.1007/s42995-023-00181-2. eCollection 2023 Aug. Mar Life Sci Technol. 2023. PMID: 37637255 Free PMC article.
-
Metagenomic Insights Reveal the Microbial Diversity and Associated Algal-Polysaccharide-Degrading Enzymes on the Surface of Red Algae among Remote Regions.Int J Mol Sci. 2023 Jul 3;24(13):11019. doi: 10.3390/ijms241311019. Int J Mol Sci. 2023. PMID: 37446198 Free PMC article.
-
Biochemical characterization and elucidation of the action mode of a GH16 family κ-carrageenase for efficient preparation of carrageenan oligosaccharides.World J Microbiol Biotechnol. 2023 Jun 7;39(8):222. doi: 10.1007/s11274-023-03668-3. World J Microbiol Biotechnol. 2023. PMID: 37285044
-
A Novel κ-Carrageenase from Marine Bacterium Rhodopirellula sallentina SM41: Heterologous Expression, Biochemical Characterization and Salt-Tolerance Mechanism Investigation.Mar Drugs. 2022 Dec 16;20(12):783. doi: 10.3390/md20120783. Mar Drugs. 2022. PMID: 36547930 Free PMC article.
References
-
- Abad L. V., Kudo H., Saiki S., Nagasawa N., Tamada M., Katsumura Y., et al. (2009). Radiation degradation studies of carrageenans. Carbohydr. Polym. 78 100–106. 10.1016/j.carbpol.2009.04.009 - DOI
-
- Barbeyron T., Zonta E., Panse S. L., Duchaud E., Michel G. (2019). Alteromonas fortis sp. nov. a non-flagellated bacterium specialized in the degradation of iota-carrageenan, and emended description of the genus Alteromonas. Int. J. Syst. Evol. Microbiol. 69 2514–2521. 10.1099/ijsem.0.003533 - DOI - PubMed
-
- Boulho R., Marty C., Freile-Pelegrín Y., Robledo D., Bourgougnon N., Bedoux G. (2017). Antiherpetic (HSV-1) activity of carrageenans from the red seaweed Solieriachordalis (Rhodophyta, Gigartinales) extracted by microwave-assisted extraction (MAE). J. Appl. Phycol. 29 2219–2228. 10.1007/s10811-017-1192-5 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous


