Experimental evidence for the existence of a second partially-ordered phase of ice VI
- PMID: 33602936
- PMCID: PMC7893076
- DOI: 10.1038/s41467-021-21351-9
Experimental evidence for the existence of a second partially-ordered phase of ice VI
Erratum in
-
Author Correction: Experimental evidence for the existence of a second partially-ordered phase of ice VI.Nat Commun. 2021 Mar 15;12(1):1800. doi: 10.1038/s41467-021-22085-4. Nat Commun. 2021. PMID: 33723240 Free PMC article. No abstract available.
Abstract
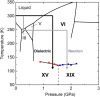
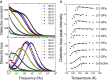
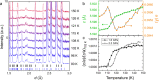
Ice exhibits extraordinary structural variety in its polymorphic structures. The existence of a new form of diversity in ice polymorphism has recently been debated in both experimental and theoretical studies, questioning whether hydrogen-disordered ice can transform into multiple hydrogen-ordered phases, contrary to the known one-to-one correspondence between disordered ice and its ordered phase. Here, we report a high-pressure phase, ice XIX, which is a second hydrogen-partially-ordered phase of ice VI. We demonstrate that disordered ice undergoes different manners of hydrogen ordering, which are thermodynamically controlled by pressure in the case of ice VI. Such multiplicity can appear in all disordered ice, and it widely provides a research approach to deepen our knowledge, for example of the crucial issues of ice: the centrosymmetry of hydrogen-ordered configurations and potentially induced (anti-)ferroelectricity. Ultimately, this research opens up the possibility of completing the phase diagram of ice.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Experiments indicating a second hydrogen ordered phase of ice VI.Chem Sci. 2018 Mar 26;9(18):4224-4234. doi: 10.1039/c8sc00135a. eCollection 2018 May 14. Chem Sci. 2018. PMID: 29780552 Free PMC article.
-
Computer simulation of hypothetical hydrogen ordered structure of ice XIX.Phys Chem Chem Phys. 2022 May 11;24(18):11023-11029. doi: 10.1039/d2cp01276f. Phys Chem Chem Phys. 2022. PMID: 35470357
-
The preparation and structures of hydrogen ordered phases of ice.Science. 2006 Mar 24;311(5768):1758-61. doi: 10.1126/science.1123896. Science. 2006. PMID: 16556840
-
Calorimetric Investigation of Hydrogen-Atom Sublattice Transitions in the Ice VI/XV/XIX Trio.J Phys Chem B. 2021 Oct 28;125(42):11777-11783. doi: 10.1021/acs.jpcb.1c07508. Epub 2021 Oct 14. J Phys Chem B. 2021. PMID: 34647740 Free PMC article.
-
Structure and nature of ice XIX.Nat Commun. 2021 May 26;12(1):3162. doi: 10.1038/s41467-021-23399-z. Nat Commun. 2021. PMID: 34039987 Free PMC article.
Cited by
-
Configurational entropy of ice XIX and its isotope effect.Sci Rep. 2024 May 7;14(1):10517. doi: 10.1038/s41598-024-61250-9. Sci Rep. 2024. PMID: 38714722 Free PMC article.
-
Revealing isochoric water nucleation: a visual study.Sci Rep. 2024 May 2;14(1):10086. doi: 10.1038/s41598-024-61053-y. Sci Rep. 2024. PMID: 38698151 Free PMC article.
-
Thermodynamically Stable Intermediate in the Course of Hydrogen Ordering from Ice V to Ice XIII.J Phys Chem Lett. 2024 Feb 1;15(4):1181-1187. doi: 10.1021/acs.jpclett.3c03411. Epub 2024 Jan 25. J Phys Chem Lett. 2024. PMID: 38270372 Free PMC article.
-
Close-Packed Ices in Nanopores.ACS Nano. 2024 Jan 9;18(1):347-354. doi: 10.1021/acsnano.3c07084. Epub 2023 Dec 18. ACS Nano. 2024. PMID: 38109520 Free PMC article.
-
Accurate crystal structure of ice VI from X-ray diffraction with Hirshfeld atom refinement.IUCrJ. 2022 Jul 16;9(Pt 5):573-579. doi: 10.1107/S2052252522006662. eCollection 2022 Sep 1. IUCrJ. 2022. PMID: 36071798 Free PMC article.
References
-
- Whalley E, Davidson DW, Heath JBR. Dielectric properties of ice VII. Ice VIII: a new phase of ice. J. Chem. Phys. 1966;45:3976–3982. doi: 10.1063/1.1727447. - DOI
-
- Whalley E. Dielectric properties of ice VII. Ice VIII: a new phase of ice. J. Chem. Phys. 1966;45:3976. doi: 10.1063/1.1727447. - DOI
-
- Jackson SM, Nield VM, Whitworth RW, Oguro M, Wilson CC. Single-crystal neutron diffraction studies of the structure of ice XI. J. Phys. Chem. B. 1997;101:6142–6145. doi: 10.1021/jp9632551. - DOI
Grants and funding
- 15H05829/MEXT | Japan Society for the Promotion of Science (JSPS)
- 18H01936/MEXT | Japan Society for the Promotion of Science (JSPS)
- 18H05224/MEXT | Japan Society for the Promotion of Science (JSPS)
- 18J13298/MEXT | Japan Society for the Promotion of Science (JSPS)
- 19H00648/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources
Other Literature Sources


