Sixteen Weeks of Supplementation with a Nutritional Quantity of a Diversity of Polyphenols from Foodstuff Extracts Improves the Health-Related Quality of Life of Overweight and Obese Volunteers: A Randomized, Double-Blind, Parallel Clinical Trial
- PMID: 33540841
- PMCID: PMC7913070
- DOI: 10.3390/nu13020492
Sixteen Weeks of Supplementation with a Nutritional Quantity of a Diversity of Polyphenols from Foodstuff Extracts Improves the Health-Related Quality of Life of Overweight and Obese Volunteers: A Randomized, Double-Blind, Parallel Clinical Trial
Abstract
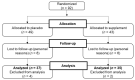
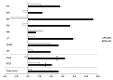
Overweight and obesity adversely affect health-related quality of life (HRQOL) through day-to-day impairments of both mental and physical functioning. It is assumed that polyphenols within the Mediterranean diet may contribute to improving HRQOL. This investigation aimed at studying the effects of a polyphenol-rich ingredient on HRQOL in overweight and obese but otherwise healthy individuals. A randomized, double-blind, placebo-controlled study including 72 volunteers was conducted. Subjects were randomly assigned to receive for a 16-week period either 900 mg/day of the supplement or a placebo. Dietary recommendations were individually determined and intakes were recorded. Daily physical mobility was also monitored. Improvement of HRQOL was set as the primary outcome and assessed at baseline and at the end of the investigation using the Short-Form 36 (SF-36) health survey. Body composition was analyzed using dual-energy X-ray absorptiometry (DXA). Physical activity was calculated using the International Physical Activity Questionnaire (IPAQ). After 16 weeks, despite there being no adherence to the Mediterranean Diet Serving Score (MDSS), supplemented individuals experienced significant HRQOL improvement (+5.3%; p = 0.001), including enhanced perceived physical (+11.2%; p = 0.002) and mental health (+4.1%; p = 0.021) components, with bodily pain, vitality, and general health being the greatest contributors. Body fat mass significantly decreased (-1.2 kg; p = 0.033), mainly within the trunk area (-1.0 kg; p = 0.002). Engagement in physical activity significantly increased (+1308 Met-min (Metabolic Equivalent Task minutes)/week; p = 0.050). Hence, chronic supplementation with nutritional diversity and dosing of a Mediterranean diet-inspired, polyphenol-rich ingredient resulted in significant amelioration in both perceived physical and mental health, concomitant with the improvement of body composition, in healthy subjects with excessive adiposity.
Keywords: Mediterranean diet; body composition; health-related quality of life; phenolic compounds; vitality.
Conflict of interest statement
Fytexia is involved in the research and development and marketing and sales of polyphenol extract-based ingredients for food and nutraceutical industries. Therefore, Fytexia has a commercial interest in this publication. UCAM and UMR 204 Nutripass were paid by Fytexia to perform and report the scientific work that formed the basis of this publication. Fytexia, UCAM, UMR 204 Nutripass, and all authors declare that the data in this report represent a true and faithful representation of the work that has been performed. The financial assistance of Fytexia is gratefully acknowledged.
Figures
Similar articles
-
Regular consumption of HolisFiit, a polyphenol-rich extract-based food supplement, improves mind and body well-being of overweight and slightly obese volunteers: a randomized, double-blind, parallel trial.Int J Food Sci Nutr. 2017 Nov;68(7):840-848. doi: 10.1080/09637486.2017.1292221. Epub 2017 Feb 23. Int J Food Sci Nutr. 2017. PMID: 28276901 Clinical Trial.
-
Regular consumption of Fiit-ns, a polyphenol extract from fruit and vegetables frequently consumed within the Mediterranean diet, improves metabolic ageing of obese volunteers: a randomized, double-blind, parallel trial.Int J Food Sci Nutr. 2015 Feb;66(1):120-5. doi: 10.3109/09637486.2014.971229. Epub 2014 Oct 31. Int J Food Sci Nutr. 2015. PMID: 25358490 Clinical Trial.
-
Effect of Arthrospira (Spirulina) maxima Supplementation and a Systematic Physical Exercise Program on the Body Composition and Cardiorespiratory Fitness of Overweight or Obese Subjects: A Double-Blind, Randomized, and Crossover Controlled Trial.Mar Drugs. 2018 Oct 1;16(10):364. doi: 10.3390/md16100364. Mar Drugs. 2018. PMID: 30275428 Free PMC article. Clinical Trial.
-
Dietary weight loss and exercise interventions effects on quality of life in overweight/obese postmenopausal women: a randomized controlled trial.Int J Behav Nutr Phys Act. 2011 Oct 25;8:118. doi: 10.1186/1479-5868-8-118. Int J Behav Nutr Phys Act. 2011. PMID: 22026966 Free PMC article. Clinical Trial.
-
Effect of Polyphenol Supplementation on Memory Functioning in Overweight and Obese Adults: A Systematic Review and Meta-Analysis.Nutrients. 2024 Feb 6;16(4):474. doi: 10.3390/nu16040474. Nutrients. 2024. PMID: 38398799 Free PMC article. Review.
Cited by
-
Dietary Strategies to Reduce Obesity Burden-Polyphenols as a Game-Changer?Healthcare (Basel). 2022 Dec 2;10(12):2430. doi: 10.3390/healthcare10122430. Healthcare (Basel). 2022. PMID: 36553954 Free PMC article.
-
European Association for the Study of Obesity Position Statement on Medical Nutrition Therapy for the Management of Overweight and Obesity in Adults Developed in Collaboration with the European Federation of the Associations of Dietitians.Obes Facts. 2023;16(1):11-28. doi: 10.1159/000528083. Epub 2022 Dec 15. Obes Facts. 2023. PMID: 36521448 Free PMC article.
-
Beneficial Effects of Anti-Inflammatory Diet in Modulating Gut Microbiota and Controlling Obesity.Nutrients. 2022 Sep 26;14(19):3985. doi: 10.3390/nu14193985. Nutrients. 2022. PMID: 36235638 Free PMC article. Review.
-
Effect of Phenolic Compounds on Human Health.Nutrients. 2021 Nov 1;13(11):3922. doi: 10.3390/nu13113922. Nutrients. 2021. PMID: 34836177 Free PMC article.
References
-
- Fayers P.M., Machin D. Quality of Life: Assessment, Analysis and Interpretation. John Wiley and Sons; Chichester, UK: 2000.
-
- WHO Noncommunicable diseases. World Health Organization. [(accessed on 7 March 2018)]; Available online: http://www.who.int/mediacentre/factsheets/fs355/en/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous


