Detection and Characterization of Bat Sarbecovirus Phylogenetically Related to SARS-CoV-2, Japan
- PMID: 33219796
- PMCID: PMC7706965
- DOI: 10.3201/eid2612.203386
Detection and Characterization of Bat Sarbecovirus Phylogenetically Related to SARS-CoV-2, Japan
Abstract
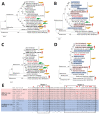
Epidemiology of bat Betacoronavirus, subgenus Sarbecovirus is largely unknown, especially outside China. We detected a sarbecovirus phylogenetically related to severe acute respiratory syndrome coronavirus 2 from Rhinolophus cornutus bats in Japan. The sarbecovirus' spike protein specifically recognizes angiotensin-converting enzyme 2 of R. cornutus, but not humans, as an entry receptor.
Keywords: COVID-19; Japan; Rhinolophus cornutus; SARS; SARS-CoV-2; bat sarbecovirus; betacoronavirus; coronavirus; coronavirus disease; respiratory infections; severe acute respiratory syndrome coronavirus 2; viruses; zoonoses.
Figures
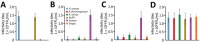
Similar articles
-
[Source of the COVID-19 pandemic: ecology and genetics of coronaviruses (Betacoronavirus: Coronaviridae) SARS-CoV, SARS-CoV-2 (subgenus Sarbecovirus), and MERS-CoV (subgenus Merbecovirus).].Vopr Virusol. 2020;65(2):62-70. doi: 10.36233/0507-4088-2020-65-2-62-70. Vopr Virusol. 2020. PMID: 32515561 Review. Russian.
-
[Etiology of epidemic outbreaks COVID-19 on Wuhan, Hubei province, Chinese People Republic associated with 2019-nCoV (Nidovirales, Coronaviridae, Coronavirinae, Betacoronavirus, Subgenus Sarbecovirus): lessons of SARS-CoV outbreak.].Vopr Virusol. 2020;65(1):6-15. doi: 10.36233/0507-4088-2020-65-1-6-15. Vopr Virusol. 2020. PMID: 32496715 Review. Russian.
-
Isolation of Bat Sarbecoviruses, Japan.Emerg Infect Dis. 2022 Dec;28(12):2500-2503. doi: 10.3201/eid2812.220801. Emerg Infect Dis. 2022. PMID: 36417954 Free PMC article.
-
In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs.J Med Virol. 2020 Oct;92(10):2105-2113. doi: 10.1002/jmv.25987. Epub 2020 May 17. J Med Virol. 2020. PMID: 32383269 Free PMC article.
-
2019_nCoV/SARS-CoV-2: rapid classification of betacoronaviruses and identification of Traditional Chinese Medicine as potential origin of zoonotic coronaviruses.Lett Appl Microbiol. 2020 May;70(5):342-348. doi: 10.1111/lam.13285. Epub 2020 Feb 28. Lett Appl Microbiol. 2020. PMID: 32060933 Free PMC article.
Cited by
-
Unveiling bat-borne viruses: a comprehensive classification and analysis of virome evolution.Microbiome. 2024 Nov 14;12(1):235. doi: 10.1186/s40168-024-01955-1. Microbiome. 2024. PMID: 39543683 Free PMC article.
-
Sarbecovirus RBD indels and specific residues dictating multi-species ACE2 adaptiveness.Nat Commun. 2024 Oct 14;15(1):8869. doi: 10.1038/s41467-024-53029-3. Nat Commun. 2024. PMID: 39402048 Free PMC article.
-
The comparison of pathogenicity among SARS-CoV-2 variants in domestic cats.Sci Rep. 2024 Sep 18;14(1):21815. doi: 10.1038/s41598-024-71791-8. Sci Rep. 2024. PMID: 39294189 Free PMC article.
-
Structural characteristics of BtKY72 RBD bound to bat ACE2 reveal multiple key residues affecting ACE2 usage of sarbecoviruses.mBio. 2024 Sep 11;15(9):e0140424. doi: 10.1128/mbio.01404-24. Epub 2024 Jul 31. mBio. 2024. PMID: 39082798 Free PMC article.
-
SARS-CoV-2-related bat viruses evade human intrinsic immunity but lack efficient transmission capacity.Nat Microbiol. 2024 Aug;9(8):2038-2050. doi: 10.1038/s41564-024-01765-z. Epub 2024 Jul 29. Nat Microbiol. 2024. PMID: 39075235
References
-
- Drexler JF, Gloza-Rausch F, Glende J, Corman VM, Muth D, Goettsche M, et al. Genomic characterization of severe acute respiratory syndrome-related coronavirus in European bats and classification of coronaviruses based on partial RNA-dependent RNA polymerase gene sequences. J Virol. 2010;84:11336–49. 10.1128/JVI.00650-10 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous


