Tissue topography steers migrating Drosophila border cells
- PMID: 33214282
- PMCID: PMC8103818
- DOI: 10.1126/science.aaz4741
Tissue topography steers migrating Drosophila border cells
Abstract
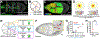
Moving cells can sense and respond to physical features of the microenvironment; however, in vivo, the significance of tissue topography is mostly unknown. Here, we used Drosophila border cells, an established model for in vivo cell migration, to study how chemical and physical information influences path selection. Although chemical cues were thought to be sufficient, live imaging, genetics, modeling, and simulations show that microtopography is also important. Chemoattractants promote predominantly posterior movement, whereas tissue architecture presents orthogonal information, a path of least resistance concentrated near the center of the egg chamber. E-cadherin supplies a permissive haptotactic cue. Our results provide insight into how cells integrate and prioritize topographical, adhesive, and chemoattractant cues to choose one path among many.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
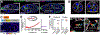
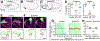

Similar articles
-
Clustered cell migration: Modeling the model system of Drosophila border cells.Semin Cell Dev Biol. 2020 Apr;100:167-176. doi: 10.1016/j.semcdb.2019.11.010. Epub 2019 Dec 11. Semin Cell Dev Biol. 2020. PMID: 31837934 Review.
-
Drosophila apc regulates delamination of invasive epithelial clusters.Dev Biol. 2012 Aug 1;368(1):76-85. doi: 10.1016/j.ydbio.2012.05.017. Epub 2012 May 22. Dev Biol. 2012. PMID: 22627290
-
Modeling and analysis of collective cell migration in an in vivo three-dimensional environment.Proc Natl Acad Sci U S A. 2016 Apr 12;113(15):E2134-41. doi: 10.1073/pnas.1522656113. Epub 2016 Mar 29. Proc Natl Acad Sci U S A. 2016. PMID: 27035964 Free PMC article.
-
A mathematical model of collective cell migration in a three-dimensional, heterogeneous environment.PLoS One. 2015 Apr 13;10(4):e0122799. doi: 10.1371/journal.pone.0122799. eCollection 2015. PLoS One. 2015. PMID: 25875645 Free PMC article.
-
[Genetic control of intercellular adhesion or how cadherins shape the fruitfly Drosophila melanogaster].Med Sci (Paris). 2007 Mar;23(3):285-90. doi: 10.1051/medsci/2007233285. Med Sci (Paris). 2007. PMID: 17349290 Review. French.
Cited by
-
Transcriptome analysis reveals temporally regulated genetic networks during Drosophila border cell collective migration.BMC Genomics. 2023 Dec 1;24(1):728. doi: 10.1186/s12864-023-09839-8. BMC Genomics. 2023. PMID: 38041052 Free PMC article.
-
Finishing the egg.Genetics. 2024 Jan 3;226(1):iyad183. doi: 10.1093/genetics/iyad183. Genetics. 2024. PMID: 38000906 Free PMC article.
-
Nuclear lamin facilitates collective border cell invasion into confined spaces in vivo.J Cell Biol. 2023 Nov 6;222(11):e202212101. doi: 10.1083/jcb.202212101. Epub 2023 Sep 11. J Cell Biol. 2023. PMID: 37695420 Free PMC article.
-
Constriction imposed by basement membrane regulates developmental cell migration.PLoS Biol. 2023 Jun 28;21(6):e3002172. doi: 10.1371/journal.pbio.3002172. eCollection 2023 Jun. PLoS Biol. 2023. PMID: 37379333 Free PMC article.
-
Septins regulate border cell surface geometry, shape, and motility downstream of Rho in Drosophila.Dev Cell. 2023 Aug 7;58(15):1399-1413.e5. doi: 10.1016/j.devcel.2023.05.017. Epub 2023 Jun 16. Dev Cell. 2023. PMID: 37329886 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases


