This is a preprint.
SARS-CoV-2 Nsp1 suppresses host but not viral translation through a bipartite mechanism
- PMID: 32995777
- PMCID: PMC7523103
- DOI: 10.1101/2020.09.18.302901
SARS-CoV-2 Nsp1 suppresses host but not viral translation through a bipartite mechanism
Update in
-
Targeting stem-loop 1 of the SARS-CoV-2 5' UTR to suppress viral translation and Nsp1 evasion.Proc Natl Acad Sci U S A. 2022 Mar 1;119(9):e2117198119. doi: 10.1073/pnas.2117198119. Proc Natl Acad Sci U S A. 2022. PMID: 35149555 Free PMC article.
Abstract
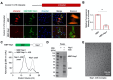
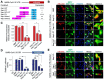
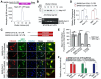
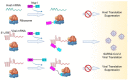
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is a highly contagious virus that underlies the current COVID-19 pandemic. SARS-CoV-2 is thought to disable various features of host immunity and cellular defense. The SARS-CoV-2 nonstructural protein 1 (Nsp1) is known to inhibit host protein translation and could be a target for antiviral therapy against COVID-19. However, how SARS-CoV-2 circumvents this translational blockage for the production of its own proteins is an open question. Here, we report a bipartite mechanism of SARS-CoV-2 Nsp1 which operates by: (1) hijacking the host ribosome via direct interaction of its C-terminal domain (CT) with the 40S ribosomal subunit and (2) specifically lifting this inhibition for SARS-CoV-2 via a direct interaction of its N-terminal domain (NT) with the 5' untranslated region (5' UTR) of SARS-CoV-2 mRNA. We show that while Nsp1-CT is sufficient for binding to 40S and inhibition of host protein translation, the 5' UTR of SARS-CoV-2 mRNA removes this inhibition by binding to Nsp1-NT, suggesting that the Nsp1-NT-UTR interaction is incompatible with the Nsp1-CT-40S interaction. Indeed, lengthening the linker between Nsp1-NT and Nsp1-CT of Nsp1 progressively reduced the ability of SARS-CoV-2 5' UTR to escape the translational inhibition, supporting that the incompatibility is likely steric in nature. The short SL1 region of the 5' UTR is required for viral mRNA translation in the presence of Nsp1. Thus, our data provide a comprehensive view on how Nsp1 switches infected cells from host mRNA translation to SARS-CoV-2 mRNA translation, and that Nsp1 and 5' UTR may be targeted for anti-COVID-19 therapeutics.
Conflict of interest statement
DECLARATION OF INTERESTS The authors declare no competing interests.
Figures

Similar articles
-
Targeting stem-loop 1 of the SARS-CoV-2 5' UTR to suppress viral translation and Nsp1 evasion.Proc Natl Acad Sci U S A. 2022 Mar 1;119(9):e2117198119. doi: 10.1073/pnas.2117198119. Proc Natl Acad Sci U S A. 2022. PMID: 35149555 Free PMC article.
-
Dynamic competition between SARS-CoV-2 NSP1 and mRNA on the human ribosome inhibits translation initiation.Proc Natl Acad Sci U S A. 2021 Feb 9;118(6):e2017715118. doi: 10.1073/pnas.2017715118. Proc Natl Acad Sci U S A. 2021. PMID: 33479166 Free PMC article.
-
Clinically observed deletions in SARS-CoV-2 Nsp1 affect its stability and ability to inhibit translation.FEBS Lett. 2022 May;596(9):1203-1213. doi: 10.1002/1873-3468.14354. Epub 2022 Apr 25. FEBS Lett. 2022. PMID: 35434785 Free PMC article.
-
Mechanisms of Coronavirus Nsp1-Mediated Control of Host and Viral Gene Expression.Cells. 2021 Feb 2;10(2):300. doi: 10.3390/cells10020300. Cells. 2021. PMID: 33540583 Free PMC article. Review.
-
Antiviral responses versus virus-induced cellular shutoff: a game of thrones between influenza A virus NS1 and SARS-CoV-2 Nsp1.Front Cell Infect Microbiol. 2024 Feb 5;14:1357866. doi: 10.3389/fcimb.2024.1357866. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38375361 Free PMC article. Review.
References
-
- Ameismeier M., Cheng J., Berninghausen O., and Beckmann R. (2018). Visualizing late states of human 40S ribosomal subunit maturation. Nature 558, 249–253. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

