NeuroMark: An automated and adaptive ICA based pipeline to identify reproducible fMRI markers of brain disorders
- PMID: 32961402
- PMCID: PMC7509081
- DOI: 10.1016/j.nicl.2020.102375
NeuroMark: An automated and adaptive ICA based pipeline to identify reproducible fMRI markers of brain disorders
Abstract
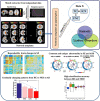
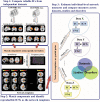
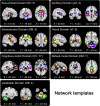
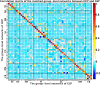
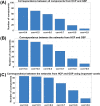
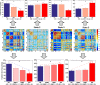
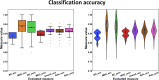
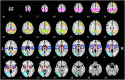
Many mental illnesses share overlapping or similar clinical symptoms, confounding the diagnosis. It is important to systematically characterize the degree to which unique and similar changing patterns are reflective of brain disorders. Increasing sharing initiatives on neuroimaging data have provided unprecedented opportunities to study brain disorders. However, it is still an open question on replicating and translating findings across studies. Standardized approaches for capturing reproducible and comparable imaging markers are greatly needed. Here, we propose a pipeline based on the priori-driven independent component analysis, NeuroMark, which is capable of estimating brain functional network measures from functional magnetic resonance imaging (fMRI) data that can be used to link brain network abnormalities among different datasets, studies, and disorders. NeuroMark automatically estimates features adaptable to each individual subject and comparable across datasets/studies/disorders by taking advantage of the reliable brain network templates extracted from 1828 healthy controls as guidance. Four studies including 2442 subjects were conducted spanning six brain disorders (schizophrenia, autism spectrum disorder, mild cognitive impairment, Alzheimer's disease, bipolar disorder, and major depressive disorder) to evaluate validity of the proposed pipeline from different perspectives (replication of brain abnormalities, cross-study comparison, identification of subtle brain changes, and multi-disorder classification using identified biomarkers). Our results highlight that NeuroMark effectively identified replicated brain network abnormalities of schizophrenia across different datasets; revealed interesting neural clues on the overlap and specificity between autism and schizophrenia; demonstrated brain functional impairments present to varying degrees in mild cognitive impairments and Alzheimer's disease; and captured biomarkers that achieved good performance in classifying bipolar disorder and major depressive disorder.
Keywords: Brain disorders; Independent component analysis; NeuroMark; Reproducible and comparable biomarkers; fMRI.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Searching Reproducible Brain Features using NeuroMark: Templates for Different Age Populations and Imaging Modalities.Neuroimage. 2024 Apr 15;292:120617. doi: 10.1016/j.neuroimage.2024.120617. Epub 2024 Apr 16. Neuroimage. 2024. PMID: 38636639 Free PMC article.
-
Federated Analysis in COINSTAC Reveals Functional Network Connectivity and Spectral Links to Smoking and Alcohol Consumption in Nearly 2,000 Adolescent Brains.Neuroinformatics. 2023 Apr;21(2):287-301. doi: 10.1007/s12021-022-09604-4. Epub 2022 Nov 25. Neuroinformatics. 2023. PMID: 36434478
-
Transdiagnostic Prediction of Affective, Cognitive, and Social Function Through Brain Reward Anticipation in Schizophrenia, Bipolar Disorder, Major Depression, and Autism Spectrum Diagnoses.Schizophr Bull. 2020 Apr 10;46(3):592-602. doi: 10.1093/schbul/sbz075. Schizophr Bull. 2020. PMID: 31586408 Free PMC article.
-
A survey on applications and analysis methods of functional magnetic resonance imaging for Alzheimer's disease.J Neurosci Methods. 2019 Apr 1;317:121-140. doi: 10.1016/j.jneumeth.2018.12.012. Epub 2018 Dec 26. J Neurosci Methods. 2019. PMID: 30593787 Review.
-
Anomalous brain gyrification patterns in major psychiatric disorders: a systematic review and transdiagnostic integration.Transl Psychiatry. 2021 Mar 17;11(1):176. doi: 10.1038/s41398-021-01297-8. Transl Psychiatry. 2021. PMID: 33731700 Free PMC article. Review.
Cited by
-
White matter functional networks in the developing brain.Front Neurosci. 2024 Oct 23;18:1467446. doi: 10.3389/fnins.2024.1467446. eCollection 2024. Front Neurosci. 2024. PMID: 39507802 Free PMC article.
-
Privacy-Preserving Visualization of Brain Functional Connectivity.bioRxiv [Preprint]. 2024 Oct 15:2024.10.11.617267. doi: 10.1101/2024.10.11.617267. bioRxiv. 2024. PMID: 39464157 Free PMC article. Preprint.
-
Addressing inconsistency in functional neuroimaging: A replicable data-driven multi-scale functional atlas for canonical brain networks.bioRxiv [Preprint]. 2024 Sep 10:2024.09.09.612129. doi: 10.1101/2024.09.09.612129. bioRxiv. 2024. PMID: 39314443 Free PMC article. Preprint.
-
Effects of connectivity hyperalignment (CHA) on estimated brain network properties: from coarse-scale to fine-scale.bioRxiv [Preprint]. 2024 Aug 28:2024.08.27.609817. doi: 10.1101/2024.08.27.609817. bioRxiv. 2024. PMID: 39253413 Free PMC article. Preprint.
-
Local-structure-preservation and redundancy-removal-based feature selection method and its application to the identification of biomarkers for schizophrenia.Neuroimage. 2024 Oct 1;299:120839. doi: 10.1016/j.neuroimage.2024.120839. Epub 2024 Sep 7. Neuroimage. 2024. PMID: 39251116 Free PMC article.
References
-
- Andreasen N.C., Paradiso S., O'Leary D.S. Cognitive dysmetria“ as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr. Bull. 1998;24(2):203–218. - PubMed
-
- Bailey A., Luthert P., Dean A., Harding B., Janota I., Montgomery M., Rutter M., Lantos P. A clinicopathological study of autism. Brain. 1998;121(Pt 5):889–905. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous


