Bacterial chemolithoautotrophy via manganese oxidation
- PMID: 32669693
- PMCID: PMC7802741
- DOI: 10.1038/s41586-020-2468-5
Bacterial chemolithoautotrophy via manganese oxidation
Abstract
Manganese is one of the most abundant elements on Earth. The oxidation of manganese has long been theorized1-yet has not been demonstrated2-4-to fuel the growth of chemolithoautotrophic microorganisms. Here we refine an enrichment culture that exhibits exponential growth dependent on Mn(II) oxidation to a co-culture of two microbial species. Oxidation required viable bacteria at permissive temperatures, which resulted in the generation of small nodules of manganese oxide with which the cells associated. The majority member of the culture-which we designate 'Candidatus Manganitrophus noduliformans'-is affiliated to the phylum Nitrospirae (also known as Nitrospirota), but is distantly related to known species of Nitrospira and Leptospirillum. We isolated the minority member, a betaproteobacterium that does not oxidize Mn(II) alone, and designate it Ramlibacter lithotrophicus. Stable-isotope probing revealed 13CO2 fixation into cellular biomass that was dependent upon Mn(II) oxidation. Transcriptomic analysis revealed candidate pathways for coupling extracellular manganese oxidation to aerobic energy conservation and autotrophic CO2 fixation. These findings expand the known diversity of inorganic metabolisms that support life, and complete a biogeochemical energy cycle for manganese5,6 that may interface with other major global elemental cycles.
Conflict of interest statement
Figures
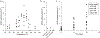


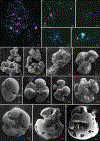


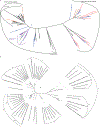



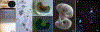

Similar articles
-
Comparative Genomics on Cultivated and Uncultivated Freshwater and Marine "Candidatus Manganitrophaceae" Species Implies Their Worldwide Reach in Manganese Chemolithoautotrophy.mBio. 2022 Apr 26;13(2):e0342121. doi: 10.1128/mbio.03421-21. Epub 2022 Mar 14. mBio. 2022. PMID: 35285693 Free PMC article.
-
Population structure of manganese-oxidizing bacteria in stratified soils and properties of manganese oxide aggregates under manganese-complex medium enrichment.PLoS One. 2013 Sep 12;8(9):e73778. doi: 10.1371/journal.pone.0073778. eCollection 2013. PLoS One. 2013. PMID: 24069232 Free PMC article.
-
Bacteriogenic manganese oxides.Acc Chem Res. 2010 Jan 19;43(1):2-9. doi: 10.1021/ar800232a. Acc Chem Res. 2010. PMID: 19778036 Review.
-
[Formation and reactions of biogenic manganese oxides with heavy metals in environment].Huan Jing Ke Xue. 2009 Feb 15;30(2):574-82. Huan Jing Ke Xue. 2009. PMID: 19402518 Chinese.
-
Microbial manganese oxide formation and interaction with toxic metal ions.J Biosci Bioeng. 2007 Jul;104(1):1-8. doi: 10.1263/jbb.104.1. J Biosci Bioeng. 2007. PMID: 17697976 Review.
Cited by
-
Nitrate-dependent antimony oxidase in an uncultured Symbiobacteriaceae member.ISME J. 2024 Jan 8;18(1):wrae204. doi: 10.1093/ismejo/wrae204. ISME J. 2024. PMID: 39413245 Free PMC article.
-
Microbial acidification by N, S, Fe and Mn oxidation as a key mechanism for deterioration of subsea tunnel sprayed concrete.Sci Rep. 2024 Sep 30;14(1):22742. doi: 10.1038/s41598-024-73911-w. Sci Rep. 2024. PMID: 39349736 Free PMC article.
-
Isolation, characterization, and genetic manipulation of cold-tolerant, manganese-oxidizing Pseudomonas sp. strains.Appl Environ Microbiol. 2024 Sep 18;90(9):e0051024. doi: 10.1128/aem.00510-24. Epub 2024 Aug 30. Appl Environ Microbiol. 2024. PMID: 39212379 Free PMC article.
-
Advances in Research on Bacterial Oxidation of Mn(II): A Visualized Bibliometric Analysis Based on CiteSpace.Microorganisms. 2024 Aug 7;12(8):1611. doi: 10.3390/microorganisms12081611. Microorganisms. 2024. PMID: 39203453 Free PMC article. Review.
-
The potential linkage between sediment oxygen demand and microbes and its contribution to the dissolved oxygen depletion in the Gan River.Front Microbiol. 2024 Jul 31;15:1413447. doi: 10.3389/fmicb.2024.1413447. eCollection 2024. Front Microbiol. 2024. PMID: 39144217 Free PMC article.
References
-
- Beijerinck M Oxydation des mangancarbonates durch Bakterien und Schimmelpilze. Folia Microbiol Delft (1913).
-
- Nealson KH, Tebo BM & Rosson RA Occurrence and mechanisms of microbial oxidation of manganese. Adv. Appl. Microbiol 33, 279–318 (1988).
-
- Tebo BM, Johnson HA, McCarthy JK & Templeton AS Geomicrobiology of manganese(II) oxidation. Trends Microbiol 13, 421–428 (2005). - PubMed
-
- Hansel C & Learman DR Geomicrobiology of manganese in Ehrlich’s Geomicrobiology (eds. Ehrlich HL, Newman DK & Kappler A) 401–452 (CRC Press, 2015). 10.1201/b19121. - DOI
-
- Myers CR & Nealson KH Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240, 1319–1321 (1988). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources


