Salience Network Atrophy Links Neuron Type-Specific Pathobiology to Loss of Empathy in Frontotemporal Dementia
- PMID: 32500143
- PMCID: PMC7566683
- DOI: 10.1093/cercor/bhaa119
Salience Network Atrophy Links Neuron Type-Specific Pathobiology to Loss of Empathy in Frontotemporal Dementia
Abstract
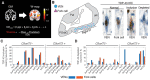
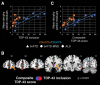
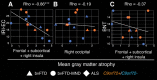
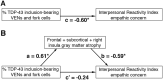
Each neurodegenerative syndrome reflects a stereotyped pattern of cellular, regional, and large-scale brain network degeneration. In behavioral variant of frontotemporal dementia (bvFTD), a disorder of social-emotional function, von Economo neurons (VENs), and fork cells are among the initial neuronal targets. These large layer 5 projection neurons are concentrated in the anterior cingulate and frontoinsular (FI) cortices, regions that anchor the salience network, a large-scale system linked to social-emotional function. Here, we studied patients with bvFTD, amyotrophic lateral sclerosis (ALS), or both, given that these syndromes share common pathobiological and genetic factors. Our goal was to determine how neuron type-specific TAR DNA-binding protein of 43 kDa (TDP-43) pathobiology relates to atrophy in specific brain structures and to loss of emotional empathy, a cardinal feature of bvFTD. We combined questionnaire-based empathy assessments, in vivo structural MR imaging, and quantitative histopathological data from 16 patients across the bvFTD/ALS spectrum. We show that TDP-43 pathobiology within right FI VENs and fork cells is associated with salience network atrophy spanning insular, medial frontal, and thalamic regions. Gray matter degeneration within these structures mediated loss of emotional empathy, suggesting a chain of influence linking the cellular, regional/network, and behavioral levels in producing signature bvFTD clinical features.
Keywords: empathy; frontoinsular cortex; frontotemporal dementia; salience network; von Economo neurons.
© The Author(s) 2020. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Similar articles
-
Neurons selectively targeted in frontotemporal dementia reveal early stage TDP-43 pathobiology.Acta Neuropathol. 2019 Jan;137(1):27-46. doi: 10.1007/s00401-018-1942-8. Epub 2018 Dec 3. Acta Neuropathol. 2019. PMID: 30511086 Free PMC article.
-
Selective frontoinsular von Economo neuron and fork cell loss in early behavioral variant frontotemporal dementia.Cereb Cortex. 2012 Feb;22(2):251-9. doi: 10.1093/cercor/bhr004. Epub 2011 Jun 8. Cereb Cortex. 2012. PMID: 21653702 Free PMC article.
-
von Economo Neuron Density and Thalamus Volumes in Behavioral Deficits in Frontotemporal Dementia Cases with and without a C9ORF72 Repeat Expansion.J Alzheimers Dis. 2017;58(3):701-709. doi: 10.3233/JAD-170002. J Alzheimers Dis. 2017. PMID: 28482638
-
Selective functional, regional, and neuronal vulnerability in frontotemporal dementia.Curr Opin Neurol. 2008 Dec;21(6):701-7. doi: 10.1097/WCO.0b013e3283168e2d. Curr Opin Neurol. 2008. PMID: 18989116 Free PMC article. Review.
-
Emotional and behavioral symptoms in neurodegenerative disease: a model for studying the neural bases of psychopathology.Annu Rev Clin Psychol. 2014;10:581-606. doi: 10.1146/annurev-clinpsy-032813-153653. Epub 2014 Jan 15. Annu Rev Clin Psychol. 2014. PMID: 24437433 Free PMC article. Review.
Cited by
-
Stress-induced cortisol response predicts empathy for pain: The role of task-based connectivity between the insula and sensorimotor cortex during acute stress.Neurobiol Stress. 2024 Oct 18;33:100682. doi: 10.1016/j.ynstr.2024.100682. eCollection 2024 Nov. Neurobiol Stress. 2024. PMID: 39502834 Free PMC article.
-
Heightened emotion processing as a compensatory mechanism in persons with Alzheimer's disease: Psychological insights from the tri-network model.Front Dement. 2022 Sep 30;1:983331. doi: 10.3389/frdem.2022.983331. eCollection 2022. Front Dement. 2022. PMID: 39081476 Free PMC article. Review.
-
Frontotemporal lobar degeneration targets brain regions linked to expression of recently evolved genes.Brain. 2024 Sep 3;147(9):3032-3047. doi: 10.1093/brain/awae205. Brain. 2024. PMID: 38940350 Free PMC article.
-
Disinhibition in dementia related to reduced morphometric similarity of cognitive control network.Brain Commun. 2024 Apr 16;6(2):fcae124. doi: 10.1093/braincomms/fcae124. eCollection 2024. Brain Commun. 2024. PMID: 38665960 Free PMC article.
-
Longitudinal cerebral perfusion in presymptomatic genetic frontotemporal dementia: GENFI results.Alzheimers Dement. 2024 May;20(5):3525-3542. doi: 10.1002/alz.13750. Epub 2024 Apr 16. Alzheimers Dement. 2024. PMID: 38623902 Free PMC article. Italian.
References
-
- Ashburner J, Friston KJ. 2000. Voxel-based morphometry - the methods. Neuroimage. 11:805–821. - PubMed
-
- Baron RM, Kenny DA. 1986. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 51:1173–1182. - PubMed
-
- Boccardi M, Laakso MP, Bresciani L, Galluzzi S, Geroldi C, Beltramello A, Soininen H, Frisoni GB. 2003. The MRI pattern of frontal and temporal brain atrophy in fronto-temporal dementia. Neurobiol Aging. 24:95–103. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous


