CT-based radiomics scores predict response to neoadjuvant chemotherapy and survival in patients with gastric cancer
- PMID: 32450841
- PMCID: PMC7249312
- DOI: 10.1186/s12885-020-06970-7
CT-based radiomics scores predict response to neoadjuvant chemotherapy and survival in patients with gastric cancer
Abstract
Background: Neoadjuvant chemotherapy is a promising treatment option for potential resectable gastric cancer, but patients' responses vary. We aimed to develop and validate a radiomics score (rad_score) to predict treatment response to neoadjuvant chemotherapy and to investigate its efficacy in survival stratification.
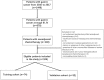
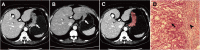
Methods: A total of 106 patients with neoadjuvant chemotherapy before gastrectomy were included (training cohort: n = 74; validation cohort: n = 32). Radiomics features were extracted from the pre-treatment portal venous-phase CT. After feature reduction, a rad_score was established by Randomised Tree algorithm. A rad_clinical_score was constructed by integrating the rad_score with clinical variables, so was a clinical score by clinical variables only. The three scores were validated regarding their discrimination and clinical usefulness. The patients were stratified into two groups according to the score thresholds (updated with post-operative clinical variables), and their survivals were compared.
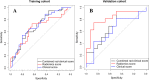
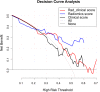
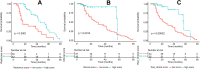
Results: In the validation cohort, the rad_score demonstrated a good predicting performance in treatment response to the neoadjuvant chemotherapy (AUC [95% CI] =0.82 [0.67, 0.98]), which was better than the clinical score (based on pre-operative clinical variables) without significant difference (0.62 [0.42, 0.83], P = 0.09). The rad_clinical_score could not further improve the performance of the rad_score (0.70 [0.51, 0.88], P = 0.16). Based on the thresholds of these scores, the high-score groups all achieved better survivals than the low-score groups in the whole cohort (all P < 0.001).
Conclusion: The rad_score that we developed was effective in predicting treatment response to neoadjuvant chemotherapy and in stratifying patients with gastric cancer into different survival groups. Our proposed strategy is useful for individualised treatment planning.
Keywords: Neoadjuvant therapy; Stomach neoplasms; Tomography, X-ray computed.
Conflict of interest statement
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Figures
Similar articles
-
Computed tomography-based radiomics nomogram for predicting therapeutic response to neoadjuvant chemotherapy in locally advanced gastric cancer : A scale for treatment predicting.Clin Transl Oncol. 2024 Aug;26(8):1944-1955. doi: 10.1007/s12094-024-03417-4. Epub 2024 Mar 11. Clin Transl Oncol. 2024. PMID: 38467894
-
Development and Validation of a Computed Tomography-Based Radiomics Signature to Predict Response to Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer.JAMA Netw Open. 2021 Aug 2;4(8):e2121143. doi: 10.1001/jamanetworkopen.2021.21143. JAMA Netw Open. 2021. PMID: 34410397 Free PMC article.
-
Multi-modal radiomics model to predict treatment response to neoadjuvant chemotherapy for locally advanced rectal cancer.World J Gastroenterol. 2020 May 21;26(19):2388-2402. doi: 10.3748/wjg.v26.i19.2388. World J Gastroenterol. 2020. PMID: 32476800 Free PMC article.
-
Neoadjuvant chemotherapy for gastric cancer. Is it a must or a fake?World J Gastroenterol. 2018 Jan 14;24(2):274-289. doi: 10.3748/wjg.v24.i2.274. World J Gastroenterol. 2018. PMID: 29375213 Free PMC article. Review.
-
Prediction of response and prognosis by a score including only pretherapeutic parameters in 410 neoadjuvant treated gastric cancer patients.Recent Results Cancer Res. 2012;196:269-89. doi: 10.1007/978-3-642-31629-6_18. Recent Results Cancer Res. 2012. PMID: 23129380 Review.
Cited by
-
Computed tomography-based radiomic model for the prediction of neoadjuvant immunochemotherapy response in patients with advanced gastric cancer.World J Gastrointest Oncol. 2024 Oct 15;16(10):4115-4128. doi: 10.4251/wjgo.v16.i10.4115. World J Gastrointest Oncol. 2024. PMID: 39473942 Free PMC article.
-
Deep learning or radiomics based on CT for predicting the response of gastric cancer to neoadjuvant chemotherapy: a meta-analysis and systematic review.Front Oncol. 2024 Mar 27;14:1363812. doi: 10.3389/fonc.2024.1363812. eCollection 2024. Front Oncol. 2024. PMID: 38601765 Free PMC article.
-
A multi-institutional study to predict the benefits of DEB-TACE and molecular targeted agent sequential therapy in unresectable hepatocellular carcinoma using a radiological-clinical nomogram.Radiol Med. 2024 Jan;129(1):14-28. doi: 10.1007/s11547-023-01736-0. Epub 2023 Oct 20. Radiol Med. 2024. PMID: 37863847
-
Artificial intelligence applications in computed tomography in gastric cancer: a narrative review.Transl Cancer Res. 2023 Sep 30;12(9):2379-2392. doi: 10.21037/tcr-23-201. Epub 2023 Aug 28. Transl Cancer Res. 2023. PMID: 37859746 Free PMC article. Review.
-
Imaging advances in efficacy assessment of gastric cancer neoadjuvant chemotherapy.Abdom Radiol (NY). 2023 Dec;48(12):3661-3676. doi: 10.1007/s00261-023-04046-1. Epub 2023 Oct 3. Abdom Radiol (NY). 2023. PMID: 37787962 Review.
References
-
- Sano T, Sasako M, Yamamoto S, et al. Cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing d2 and extended Para-aortic lymphadenectomy—Japan clinical oncology group study 9501. J Clin Oncol. 2004;22:2767–2773. doi: 10.1200/JCO.2004.10.184. - DOI - PubMed
MeSH terms
Grants and funding
- 81672343 and 81871915/National Natural Foundation of China
- 2017A030313570/Natural Science Foundation of Guangdong Province
- 2018A030310326/Natural Science Foundation of Guangdong Province
- 2018A030310282/Natural Science Foundation of Guangdong Province
- 201607010050/Science and Technology Program of Guangzhou, China
LinkOut - more resources
Full Text Sources
Medical


