Nilotinib Effects on Safety, Tolerability, and Potential Biomarkers in Parkinson Disease: A Phase 2 Randomized Clinical Trial
- PMID: 31841599
- PMCID: PMC6990742
- DOI: 10.1001/jamaneurol.2019.4200
Nilotinib Effects on Safety, Tolerability, and Potential Biomarkers in Parkinson Disease: A Phase 2 Randomized Clinical Trial
Abstract
Importance: This study evaluated nilotinib safety and its effects on biomarkers as a potential disease-modifying drug in Parkinson disease.
Objectives: To assess nilotinib effects on safety and pharmacokinetics and measure the change in exploratory biomarkers in patients with moderately severe Parkinson disease.
Design, setting, and participants: This was a single-center, phase 2, randomized, double-blind, placebo-controlled trial with 300 patients approached in clinic; of these, 200 declined to participate, 100 were screened, 25 were excluded, and 75 were randomized 1:1:1 into placebo; nilotinib, 150-mg; or nilotinib, 300-mg groups. Recruitment started on May 17, 2017, and ended April 28, 2018, and follow-up ended August 10, 2019. Parkinson disease was confirmed according to the UK Brain Bank diagnostic criteria and symptoms were stabilized with use of optimal levodopa and/or dopamine agonists and other medications used in Parkinson disease.
Interventions: Nilotinib vs placebo, administered orally once daily for 12 months followed by a 3-month washout period.
Main outcomes and measures: It was hypothesized that nilotinib is safe and can be detected in the cerebrospinal fluid, where it alters exploratory biomarkers via inhibition of Abelson tyrosine kinase and potentially improves clinical outcomes.
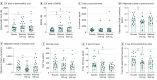
Results: Of the 75 patients included in the study, 55 were men (73.3%); mean (SD) age was 68.4 (8.2) years. Doses of 150 or 300 mg of nilotinib were reasonably safe, although more serious adverse events were detected in the nilotinib (150 mg: 6 [24%]; 300 mg: 12 [48%]) vs placebo (4 [16%]) groups. The 150-mg nilotinib group showed an increase in cerebrospinal fluid levels of the dopamine metabolites homovanillic acid (159.80nM; 90% CI, 7.04-312.60nM; P = .04) and 3,4-dihydroxyphenylacetic acid (4.87nM; 90% CI, 1.51-8.23nM; P = .01), and the 300-mg nilotinib group showed an increase in 3,4-dihydroxyphenylacetic acid (7.52nM; 90% CI, 2.35-12.69nM; P = .01). The nilotinib 150-mg but not the nilotinib 300-mg group demonstrated a reduction of α-synuclein oligomers (-0.04 pg/mL; 90% CI, -0.08 to 0.01 pg/mL; P = .03). A significant reduction of hyperphosphorylated tau levels was seen in the nilotinib 150-mg (-10.04 pg/mL; 90% CI, -17.41 to -2.67 pg/mL; P = .01) and nilotinib 300-mg (-12.05 pg/mL; 90% CI, -19.21 to -4.90 pg/mL; P = .01) groups.
Conclusions and relevance: In this study, nilotinib appeared to be reasonably safe and detectable in the cerebrospinal fluid. Exploratory biomarkers were altered in response to nilotinib. Taken together, these data will guide the development of a phase 3 study to investigate the effects of nilotinib therapy in patients with Parkinson disease.
Trial registration: ClinicalTrials.gov identifier: NCT02954978.
Conflict of interest statement
Figures
Comment in
-
The Narrowing Path for Nilotinib and Other Potential Disease-Modifying Therapies for Parkinson Disease.JAMA Neurol. 2020 Mar 1;77(3):295-297. doi: 10.1001/jamaneurol.2019.3983. JAMA Neurol. 2020. PMID: 31841588 No abstract available.
-
Dopamine Metabolite Biomarkers and Testing for Disease Modification in Parkinson Disease.JAMA Neurol. 2020 Aug 1;77(8):1038-1039. doi: 10.1001/jamaneurol.2020.1928. JAMA Neurol. 2020. PMID: 32597929 No abstract available.
-
Dopamine Metabolite Biomarkers and Testing for Disease Modification in Parkinson Disease-Reply.JAMA Neurol. 2020 Aug 1;77(8):1039-1040. doi: 10.1001/jamaneurol.2020.1931. JAMA Neurol. 2020. PMID: 32597985 No abstract available.
Similar articles
-
Efficacy of Nilotinib in Patients With Moderately Advanced Parkinson Disease: A Randomized Clinical Trial.JAMA Neurol. 2021 Mar 1;78(3):312-320. doi: 10.1001/jamaneurol.2020.4725. JAMA Neurol. 2021. PMID: 33315105 Free PMC article. Clinical Trial.
-
Pharmacokinetics and pharmacodynamics of a single dose Nilotinib in individuals with Parkinson's disease.Pharmacol Res Perspect. 2019 Mar 12;7(2):e00470. doi: 10.1002/prp2.470. eCollection 2019 Apr. Pharmacol Res Perspect. 2019. PMID: 30906562 Free PMC article. Clinical Trial.
-
Nilotinib Effects on Safety, Tolerability, and Biomarkers in Alzheimer's Disease.Ann Neurol. 2020 Jul;88(1):183-194. doi: 10.1002/ana.25775. Epub 2020 May 28. Ann Neurol. 2020. PMID: 32468646 Free PMC article.
-
Nilotinib Effects in Parkinson's disease and Dementia with Lewy bodies.J Parkinsons Dis. 2016 Jul 11;6(3):503-17. doi: 10.3233/JPD-160867. J Parkinsons Dis. 2016. PMID: 27434297 Free PMC article. Clinical Trial.
-
Tolerability and safety of ropinirole versus other dopamine agonists and levodopa in the treatment of Parkinson's disease: meta-analysis of randomized controlled trials.Drug Saf. 2010 Feb 1;33(2):147-61. doi: 10.2165/11319860-000000000-00000. Drug Saf. 2010. PMID: 20082541 Review.
Cited by
-
Bioavailability as Proof to Authorize the Clinical Testing of Neurodegenerative Drugs-Protocols and Advice for the FDA to Meet the ALS Act Vision.Int J Mol Sci. 2024 Sep 23;25(18):10211. doi: 10.3390/ijms251810211. Int J Mol Sci. 2024. PMID: 39337696 Free PMC article. Review.
-
Trends on Novel Targets and Nanotechnology-Based Drug Delivery System in the Treatment of Parkinson's disease: Recent Advancement in Drug Development.Curr Drug Targets. 2024;25(15):987-1011. doi: 10.2174/0113894501312703240826070530. Curr Drug Targets. 2024. PMID: 39313872 Review.
-
Tyrosine kinases: multifaceted receptors at the intersection of several neurodegenerative disease-associated processes.Front Dement. 2024 Aug 16;3:1458038. doi: 10.3389/frdem.2024.1458038. eCollection 2024. Front Dement. 2024. PMID: 39221072 Free PMC article. Review.
-
The Role of Alpha-Synuclein and Tubulin-Associated Unit (Tau) Proteins in the Diagnosis, Prognosis, and Treatment of Parkinson's Disease: A Systematic Review.Cureus. 2024 Jul 17;16(7):e64766. doi: 10.7759/cureus.64766. eCollection 2024 Jul. Cureus. 2024. PMID: 39156411 Free PMC article. Review.
-
Dual-Action Kinase Inhibitors Influence p38α MAP Kinase Dephosphorylation.bioRxiv [Preprint]. 2024 Aug 8:2024.05.15.594272. doi: 10.1101/2024.05.15.594272. bioRxiv. 2024. PMID: 39149408 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous


