Effects of acidosis on neuronal voltage-gated sodium channels: Nav1.1 and Nav1.3
- PMID: 30362397
- PMCID: PMC6284583
- DOI: 10.1080/19336950.2018.1539611
Effects of acidosis on neuronal voltage-gated sodium channels: Nav1.1 and Nav1.3
Abstract
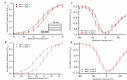
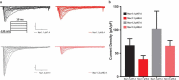
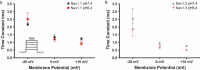
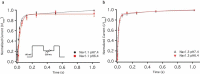
Voltage-gated sodium channels are key contributors to membrane excitability. These channels are expressed in a tissue-specific manner. Mutations and modulation of these channels underlie various physiological and pathophysiological manifestations. The effects of changes in extracellular pH on channel gating have been studied on several sodium channel subtypes. Among these, Nav1.5 is the most pH-sensitive channel, with Nav1.2 and Nav1.4 being mostly pH-resistant channels. However, pH effects have not been characterized on other sodium channel subtypes. In this study, we sought to determine whether Nav1.1 and Nav1.3 display resistance or sensitivity to changes in extracellular pH. These two sodium channel subtypes are predominantly found in inhibitory neurons. The expression of these channels highly depends on age and the developmental stage of neurons, with Nav1.3 being found mostly in neonatal neurons, and Nav1.1 being found in adult neurons. Our present results indicate that, during extracellular acidosis, both channels show a depolarization in the voltage-dependence of activation and moderate reduction in current density. Voltage-dependence of steady-state fast inactivation and recovery from fast inactivation were unchanged. We conclude that Nav1.1 and Nav1.3 have similar pH-sensitivities.
Keywords: Acidosis; Nav1.1; Nav1.3; electrophysiology; pH.
Figures

Similar articles
-
Structure and function of hainantoxin-III, a selective antagonist of neuronal tetrodotoxin-sensitive voltage-gated sodium channels isolated from the Chinese bird spider Ornithoctonus hainana.J Biol Chem. 2013 Jul 12;288(28):20392-403. doi: 10.1074/jbc.M112.426627. Epub 2013 May 23. J Biol Chem. 2013. PMID: 23703613 Free PMC article.
-
BmK AEP, an Anti-Epileptic Peptide Distinctly Affects the Gating of Brain Subtypes of Voltage-Gated Sodium Channels.Int J Mol Sci. 2019 Feb 8;20(3):729. doi: 10.3390/ijms20030729. Int J Mol Sci. 2019. PMID: 30744067 Free PMC article.
-
Effects of extracellular pH on voltage-gated Na+, K+ and Ca2+ currents in isolated rat CA1 neurons.J Physiol. 1996 Jun 15;493 ( Pt 3)(Pt 3):719-32. doi: 10.1113/jphysiol.1996.sp021417. J Physiol. 1996. PMID: 8799894 Free PMC article.
-
Voltage-gated sodium channels and pain-related disorders.Clin Sci (Lond). 2016 Dec 1;130(24):2257-2265. doi: 10.1042/CS20160041. Clin Sci (Lond). 2016. PMID: 27815510 Review.
-
The Nax (SCN7A) channel: an atypical regulator of tissue homeostasis and disease.Cell Mol Life Sci. 2021 Jul;78(14):5469-5488. doi: 10.1007/s00018-021-03854-2. Epub 2021 Jun 8. Cell Mol Life Sci. 2021. PMID: 34100980 Free PMC article. Review.
Cited by
-
Therapeutic potential of orally applied KB-R7943 in streptozotocin-induced neuropathy in rats.Heliyon. 2024 Mar 12;10(6):e27367. doi: 10.1016/j.heliyon.2024.e27367. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38524546 Free PMC article.
-
Carbogen-Induced Respiratory Acidosis Blocks Experimental Seizures by a Direct and Specific Inhibition of NaV1.2 Channels in the Axon Initial Segment of Pyramidal Neurons.J Neurosci. 2023 Mar 8;43(10):1658-1667. doi: 10.1523/JNEUROSCI.1387-22.2022. Epub 2023 Feb 2. J Neurosci. 2023. PMID: 36732074 Free PMC article.
-
Sodium channels and the ionic microenvironment of breast tumours.J Physiol. 2023 May;601(9):1543-1553. doi: 10.1113/JP282306. Epub 2022 Oct 21. J Physiol. 2023. PMID: 36183245 Free PMC article.
-
Calmodulin Interactions with Voltage-Gated Sodium Channels.Int J Mol Sci. 2021 Sep 10;22(18):9798. doi: 10.3390/ijms22189798. Int J Mol Sci. 2021. PMID: 34575961 Free PMC article. Review.
-
Cationic Modulation of Voltage-Gated Sodium Channel (Nav1.5): Neonatal Versus Adult Splice Variants-1. Monovalent (H+) Ions.Bioelectricity. 2019 Sep 1;1(3):139-147. doi: 10.1089/bioe.2019.0012. Epub 2019 Sep 16. Bioelectricity. 2019. PMID: 34471816 Free PMC article.
References
-
- Catterall WA. Voltage-gated sodium channels at 60: structure, function and pathophysiology. J Physiol [Internet]. 2012. [cited 2017 June 3];590:2577–2589. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22473783 - PMC - PubMed
-
- Ghovanloo M-R, Aimar K, Ghadiry-Tavi R, et al. Physiology and pathophysiology of sodium channel inactivation [Internet] In: Robert J. French, Sergei Yu. Noskov, editors. Current topics in membranes. Amsterdam, Netherlands: Elsevier; 2016. 479–509. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1063582316300011 - PubMed
-
- Calhoun JD, Isom LL.. The role of non-pore-forming β subunits in physiology and pathophysiology of voltage-gated sodium channels In: Peter C. Ruben, editor. Voltage gated sodium channels. New York (NY): Springer; 2014. - PubMed
-
- Estacion M, Gasser A, Dib-Hajj SD, et al. A sodium channel mutation linked to epilepsy increases ramp and persistent current of Nav1.3 and induces hyperexcitability in hippocampal neurons. Exp Neurol. 2010;224:362–368. - PubMed
-
- Cannon SC. Pathomechanisms in channelopathies of skeletal muscle and brain. Annu Rev Neurosci [Internet]. 2006;29:387–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

