Gene Therapy for Parkinson's Disease, An Update
- PMID: 29710735
- PMCID: PMC6027861
- DOI: 10.3233/JPD-181331
Gene Therapy for Parkinson's Disease, An Update
Abstract
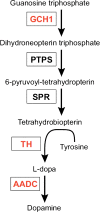
The current mainstay treatment of Parkinson's disease (PD) consists of dopamine replacement therapy which, in addition to causing several side effects, does not delay disease progression. The field of gene therapy offers a potential means to improve current therapy. The present review gives an update of the present status of gene therapy for PD. Both non-disease and disease modifying transgenes have been tested for PD gene therapy in animal and human studies. Non-disease modifying treatments targeting dopamine or GABA synthesis have been successful and promising at improving PD symptomatology in randomized clinical studies, but substantial testing remains before these can be implemented in the standard clinical treatment repertoire. As for disease modifying targets that theoretically offer the possibility of slowing the progression of disease, several neurotrophic factors show encouraging results in preclinical models (e.g., neurturin, GDNF, BDNF, CDNF, VEGF-A). However, so far, clinical trials have only tested neurturin, and, unfortunately, no trial has been able to meet its primary endpoint. Future clinical trials with neurotrophic factors clearly deserve to be conducted, considering the still enticing goal of actually slowing the disease process of PD. As alternative types of gene therapy, opto- and chemogenetics might also find future use in PD treatment and novel genome-editing technology could also potentially be applied as individualized gene therapy for genetic types of PD.
Keywords: BDNF; CDNF; GAD; GDNF; Gene therapy; MANF; NRTN; Parkinson’s disease targets; chemogenetics; dopamine; genome editing; neurotrophic factors; optogenetics.
Figures
Similar articles
-
Subthalamic GAD gene transfer in Parkinson disease patients who are candidates for deep brain stimulation.Hum Gene Ther. 2001 Aug 10;12(12):1589-91. Hum Gene Ther. 2001. PMID: 11529246 Clinical Trial.
-
Delivery of CDNF by AAV-mediated gene transfer protects dopamine neurons and regulates ER stress and inflammation in an acute MPTP mouse model of Parkinson's disease.Sci Rep. 2024 Jul 17;14(1):16487. doi: 10.1038/s41598-024-65735-5. Sci Rep. 2024. PMID: 39019902 Free PMC article.
-
From lab bench to hope: a review of gene therapies in clinical trials for Parkinson's disease and challenges.Neurol Sci. 2024 Oct;45(10):4699-4710. doi: 10.1007/s10072-024-07599-1. Epub 2024 May 25. Neurol Sci. 2024. PMID: 38795270 Review.
-
CDNF Protein Therapy in Parkinson's Disease.Cell Transplant. 2019 Apr;28(4):349-366. doi: 10.1177/0963689719840290. Epub 2019 Apr 4. Cell Transplant. 2019. PMID: 30947516 Free PMC article.
-
Prospects of Neurotrophic Factors for Parkinson's Disease: Comparison of Protein and Gene Therapy.Hum Gene Ther. 2015 Aug;26(8):550-9. doi: 10.1089/hum.2015.065. Hum Gene Ther. 2015. PMID: 26176331 Review.
Cited by
-
Trends on Novel Targets and Nanotechnology-Based Drug Delivery System in the Treatment of Parkinson's disease: Recent Advancement in Drug Development.Curr Drug Targets. 2024;25(15):987-1011. doi: 10.2174/0113894501312703240826070530. Curr Drug Targets. 2024. PMID: 39313872 Review.
-
Limitations and potential strategies of immune checkpoint blockade in age-related neurodegenerative disorders.J Physiol Sci. 2024 Sep 23;74(1):46. doi: 10.1186/s12576-024-00933-4. J Physiol Sci. 2024. PMID: 39313800 Free PMC article. Review.
-
A Comprehensive Approach to Parkinson's Disease: Addressing Its Molecular, Clinical, and Therapeutic Aspects.Int J Mol Sci. 2024 Jun 29;25(13):7183. doi: 10.3390/ijms25137183. Int J Mol Sci. 2024. PMID: 39000288 Free PMC article. Review.
-
Dopaminergic Cell Replacement for Parkinson's Disease: Addressing the Intracranial Delivery Hurdle.J Parkinsons Dis. 2024;14(3):415-435. doi: 10.3233/JPD-230328. J Parkinsons Dis. 2024. PMID: 38457149 Free PMC article. Review.
-
Can pluripotent/multipotent stem cells reverse Parkinson's disease progression?Front Neurosci. 2024 Jan 31;18:1210447. doi: 10.3389/fnins.2024.1210447. eCollection 2024. Front Neurosci. 2024. PMID: 38356648 Free PMC article. Review.
References
-
- Global Study of Disease Study 2013, Collaborators (2015) Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 386, 743–800. - PMC - PubMed
-
- Obeso JA, Rodriguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C, Rodriguez M (2008) Functional organization of the basal ganglia: Therapeutic implications for Parkinson’s disease. Mov Disord 23(Suppl 3), 548–559. - PubMed
-
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388, 839–840. - PubMed
-
- Berglund MM, Hipskind PA, Gehlert DR (2003) Recent developments in our understanding of the physiological role of PP-fold peptide receptor subtypes. Exp Biol Med 228, 217–244. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous


