Initial clinical outcomes of proton beam radiotherapy for hepatocellular carcinoma
- PMID: 29580046
- PMCID: PMC5903361
- DOI: 10.3857/roj.2017.00409
Initial clinical outcomes of proton beam radiotherapy for hepatocellular carcinoma
Abstract
Purpose: This study aimed to evaluate the initial outcomes of proton beam therapy (PBT) for hepatocellular carcinoma (HCC) in terms of tumor response and safety.
Materials and methods: HCC patients who were not indicated for standard curative local modalities and who were treated with PBT at Samsung Medical Center from January 2016 to February 2017 were enrolled. Toxicity was scored using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Tumor response was evaluated using modified Response Evaluation Criteria in Solid Tumors (mRECIST).
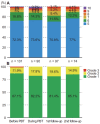
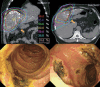
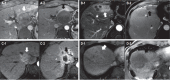
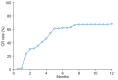
Results: A total of 101 HCC patients treated with PBT were included. Patients were treated with an equivalent dose of 62-92 GyE10. Liver function status was not significantly affected after PBT. Greater than 80% of patients had Child-Pugh class A and albumin-bilirubin (ALBI) grade 1 up to 3-months after PBT. Of 78 patients followed for three months after PBT, infield complete and partial responses were achieved in 54 (69.2%) and 14 (17.9%) patients, respectively.
Conclusion: PBT treatment of HCC patients showed a favorable infield complete response rate of 69.2% with acceptable acute toxicity. An additional follow-up study of these patients will be conducted.
Keywords: Hepatocellular carcinoma; Proton; Radiotherapy; Response; Toxicity.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

Similar articles
-
Proton beam radiotherapy combined with anti-PD1/PDL1 immune checkpoint inhibitors for advanced hepatocellular carcinoma.Am J Cancer Res. 2022 Apr 15;12(4):1606-1620. eCollection 2022. Am J Cancer Res. 2022. PMID: 35530291 Free PMC article.
-
Phase I dose-escalation study of proton beam therapy for inoperable hepatocellular carcinoma.Cancer Res Treat. 2015 Jan;47(1):34-45. doi: 10.4143/crt.2013.218. Epub 2014 Sep 11. Cancer Res Treat. 2015. PMID: 25381830 Free PMC article.
-
Proton beam stereotactic body radiotherapy and hypofractionated therapy with pencil beam scanning is safe and effective for advanced hepatocellular carcinoma and intrahepatic cholangiocarcinoma: A single center experience.J Radiosurg SBRT. 2023;9(1):43-52. J Radiosurg SBRT. 2023. PMID: 38029012 Free PMC article.
-
Proton therapy for hepatocellular carcinoma: Current knowledges and future perspectives.World J Gastroenterol. 2018 Jul 28;24(28):3090-3100. doi: 10.3748/wjg.v24.i28.3090. World J Gastroenterol. 2018. PMID: 30065555 Free PMC article. Review.
-
Proton beam therapy for hepatocellular carcinoma.Expert Rev Anticancer Ther. 2017 Oct;17(10):911-924. doi: 10.1080/14737140.2017.1368392. Epub 2017 Aug 21. Expert Rev Anticancer Ther. 2017. PMID: 28825506 Review.
Cited by
-
Proton Therapy in the Management of Hepatocellular Carcinoma.Cancers (Basel). 2022 Jun 12;14(12):2900. doi: 10.3390/cancers14122900. Cancers (Basel). 2022. PMID: 35740567 Free PMC article. Review.
-
Proton beam therapy to bridge or downstage locally advanced hepatocellular carcinoma to living donor liver transplantation.Hepatobiliary Surg Nutr. 2022 Feb;11(1):103-111. doi: 10.21037/hbsn-21-379. Hepatobiliary Surg Nutr. 2022. PMID: 35284524 Free PMC article. No abstract available.
-
A preliminary study of hepatocellular carcinoma post proton beam therapy using MRI as an early prediction of treatment effectiveness.PLoS One. 2021 Mar 23;16(3):e0249003. doi: 10.1371/journal.pone.0249003. eCollection 2021. PLoS One. 2021. PMID: 33755701 Free PMC article.
-
Do Biliary Complications after Proton Beam Therapy for Perihilar Hepatocellular Carcinoma Matter?Cancers (Basel). 2020 Aug 24;12(9):2395. doi: 10.3390/cancers12092395. Cancers (Basel). 2020. PMID: 32847035 Free PMC article.
-
Clinical Significance of Systemic Inflammation Markers in Newly Diagnosed, Previously Untreated Hepatocellular Carcinoma.Cancers (Basel). 2020 May 21;12(5):1300. doi: 10.3390/cancers12051300. Cancers (Basel). 2020. PMID: 32455607 Free PMC article.
References
-
- Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–48. - PMC - PubMed
-
- Kim BH, Lim YS, Kim EY, et al. Temporal improvement in survival of patients with hepatocellular carcinoma in a hepatitis B virus-endemic population. J Gastroenterol Hepatol. 2018;33:475–83. - PubMed
-
- Kudo M, Izumi N, Ichida T, et al. Report of the 19th follow-up survey of primary liver cancer in Japan. Hepatol Res. 2016;46:372–90. - PubMed
-
- Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–9. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources


