Nervous System Malformations
- PMID: 29432238
- PMCID: PMC6463295
- DOI: 10.1212/CON.0000000000000561
Nervous System Malformations
Abstract
Purpose of review: This article provides an overview of the most common nervous system malformations and serves as a reference for the latest advances in diagnosis and treatment.
Recent findings: Major advances have occurred in recognizing the genetic basis of nervous system malformations. Environmental causes of nervous system malformations, such as perinatal infections including Zika virus, are also reviewed. Treatment for nervous system malformations begins prior to birth with prevention. Folic acid supplementation reduces the risk of neural tube defects and is an important part of health maintenance for pregnant women. Fetal surgery is now available for prenatal repair of myelomeningocele and has been demonstrated to improve outcomes.
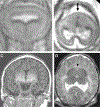
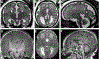
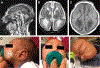
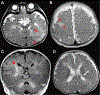
Summary: Each type of nervous system malformation is relatively uncommon, but, collectively, they constitute a large population of neurologic patients. The diagnosis of nervous system malformations begins with radiographic characterization. Genetic studies, including chromosomal microarray, targeted gene sequencing, and next-generation sequencing, are increasingly important aspects of the assessment. A genetic diagnosis may identify an associated medical condition and is necessary for family planning. Treatment consists primarily of supportive therapies for developmental delays and epilepsy, but prenatal surgery for myelomeningocele offers a glimpse of future possibilities. Prognosis depends on multiple clinical factors, including the examination findings, imaging characteristics, and genetic results. Treatment is best conducted in a multidisciplinary setting with neurology, neurosurgery, developmental pediatrics, and genetics working together as a comprehensive team.
Figures
Similar articles
-
Spectrum of prenatally detected central nervous system malformations: Neural tube defects continue to be the leading foetal malformation.Indian J Med Res. 2017 Apr;145(4):471-478. doi: 10.4103/ijmr.IJMR_1882_14. Indian J Med Res. 2017. PMID: 28862178 Free PMC article.
-
[Prenatal diagnosis of central nervous system malformations].Ideggyogy Sz. 2013 Jul 30;66(7-8):228-34. Ideggyogy Sz. 2013. PMID: 23971353 Hungarian.
-
[Significance of magnetic resonance studies in prenatal diagnosis of malformations of the fetal central nervous system].Orv Hetil. 2009 Jul 5;150(27):1275-80. doi: 10.1556/OH.2009.28626. Orv Hetil. 2009. PMID: 19531461 Review. Hungarian.
-
Fetal central nervous system malformations on MR images.Brain Dev. 2009 Mar;31(3):185-99. doi: 10.1016/j.braindev.2008.07.007. Epub 2008 Aug 30. Brain Dev. 2009. PMID: 18762395 Review.
-
Fetal Neurosonogaphy: Ultrasound and Magnetic Resonance Imaging in Competition.Ultraschall Med. 2016 Dec;37(6):555-557. doi: 10.1055/s-0042-117142. Epub 2016 Dec 15. Ultraschall Med. 2016. PMID: 27978593 English.
Cited by
-
Transanal irrigation to manage neurogenic bowel in the pediatric population with spina bifida: a scoping review.J Pediatr (Rio J). 2023 Jul-Aug;99(4):322-334. doi: 10.1016/j.jped.2023.02.001. Epub 2023 Feb 24. J Pediatr (Rio J). 2023. PMID: 36852756 Free PMC article. Review.
-
Pregnancy-Related Extracellular Vesicles Revisited.Int J Mol Sci. 2021 Apr 9;22(8):3904. doi: 10.3390/ijms22083904. Int J Mol Sci. 2021. PMID: 33918880 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials


