Using para hydrogen to hyperpolarize amines, amides, carboxylic acids, alcohols, phosphates, and carbonates
- PMID: 29326984
- PMCID: PMC5756661
- DOI: 10.1126/sciadv.aao6250
Using para hydrogen to hyperpolarize amines, amides, carboxylic acids, alcohols, phosphates, and carbonates
Abstract
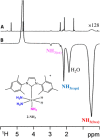
Hyperpolarization turns weak nuclear magnetic resonance (NMR) and magnetic resonance imaging (MRI) responses into strong signals, so normally impractical measurements are possible. We use parahydrogen to rapidly hyperpolarize appropriate 1H, 13C, 15N, and 31P responses of analytes (such as NH3) and important amines (such as phenylethylamine), amides (such as acetamide, urea, and methacrylamide), alcohols spanning methanol through octanol and glucose, the sodium salts of carboxylic acids (such as acetic acid and pyruvic acid), sodium phosphate, disodium adenosine 5'-triphosphate, and sodium hydrogen carbonate. The associated signal gains are used to demonstrate that it is possible to collect informative single-shot NMR spectra of these analytes in seconds at the micromole level in a 9.4-T observation field. To achieve these wide-ranging signal gains, we first use the signal amplification by reversible exchange (SABRE) process to hyperpolarize an amine or ammonia and then use their exchangeable NH protons to relay polarization into the analyte without changing its identity. We found that the 1H signal gains reach as high as 650-fold per proton, whereas for 13C, the corresponding signal gains achieved in a 1H-13C refocused insensitive nuclei enhanced by polarization transfer (INEPT) experiment exceed 570-fold and those in a direct-detected 13C measurement exceed 400-fold. Thirty-one examples are described to demonstrate the applicability of this technique.
Figures


Similar articles
-
Signal Amplification by Reversible Exchange (SABRE): From Discovery to Diagnosis.Angew Chem Int Ed Engl. 2018 Jun 4;57(23):6742-6753. doi: 10.1002/anie.201710406. Epub 2018 Apr 27. Angew Chem Int Ed Engl. 2018. PMID: 29316071 Review.
-
Hyperpolarized [13C]-2-hydroxyethylpropionate.2008 Jan 31 [updated 2008 Mar 5]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Jan 31 [updated 2008 Mar 5]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641315 Free Books & Documents. Review.
-
Application of parahydrogen induced polarization techniques in NMR spectroscopy and imaging.Acc Chem Res. 2012 Aug 21;45(8):1247-57. doi: 10.1021/ar2003094. Epub 2012 Mar 27. Acc Chem Res. 2012. PMID: 22452702
-
Direct and indirect hyperpolarisation of amines using parahydrogen.Chem Sci. 2018 Mar 9;9(15):3677-3684. doi: 10.1039/c8sc00526e. eCollection 2018 Apr 21. Chem Sci. 2018. PMID: 29780498 Free PMC article.
-
"Direct" 13 C Hyperpolarization of 13 C-Acetate by MicroTesla NMR Signal Amplification by Reversible Exchange (SABRE).Angew Chem Int Ed Engl. 2020 Jan 2;59(1):418-423. doi: 10.1002/anie.201910506. Epub 2019 Nov 14. Angew Chem Int Ed Engl. 2020. PMID: 31661580
Cited by
-
Identifying routes for transferring spin polarization from parahydrogen to protic solvents.Chem Commun (Camb). 2024 Nov 21;60(94):13923-13926. doi: 10.1039/d4cc03468f. Chem Commun (Camb). 2024. PMID: 39503617 Free PMC article.
-
Nuclear spin polarization of lactic acid via exchange of parahydrogen-polarized protons.Commun Chem. 2024 Aug 8;7(1):172. doi: 10.1038/s42004-024-01254-8. Commun Chem. 2024. PMID: 39112677 Free PMC article.
-
Modeling Ligand Exchange Kinetics in Iridium Complexes Catalyzing SABRE Nuclear Spin Hyperpolarization.Anal Chem. 2024 Jul 23;96(29):11790-11799. doi: 10.1021/acs.analchem.4c01374. Epub 2024 Jul 8. Anal Chem. 2024. PMID: 38976810 Free PMC article.
-
Zero-field J-spectroscopy of quadrupolar nuclei.Nat Commun. 2024 May 27;15(1):4487. doi: 10.1038/s41467-024-48390-2. Nat Commun. 2024. PMID: 38802356
-
Facile hyperpolarization chemistry for molecular imaging and metabolic tracking of [1-13C]pyruvate in vivo.J Magn Reson Open. 2023 Dec;16-17:100129. doi: 10.1016/j.jmro.2023.100129. Epub 2023 Jul 13. J Magn Reson Open. 2023. PMID: 38090022 Free PMC article.
References
-
- Ardenkjaer-Larsen J. H., On the present and future of dissolution-DNP. J. Magn. Reson. 264, 3–12 (2016). - PubMed
-
- Bowers C. R., Weitekamp D. P., Parahydrogen and synthesis allow dramatically enhanced nuclear alignment. J. Am. Chem. Soc. 109, 5541–5542 (1987).
-
- Natterer J., Bargon J., Parahydrogen induced polarization. Prog. Nucl. Magn. Reson. Spectrosc. 31, 293–315 (1997).
-
- Green R. A., Adams R. W., Duckett S. B., Mewis R. E., Williamson D. C., Green G. G. R., The theory and practice of hyperpolarization in magnetic resonance using parahydrogen. Prog. Nucl. Magn. Reson. Spectrosc. 67, 1–48 (2012). - PubMed
-
- Adams R. W., Aguilar J. A., Atkinson K. D., Cowley M. J., Elliott P. I. P., Duckett S. B., Green G. G. R., Khazal I. G., López-Serrano J., Williamson D. C., Reversible interactions with para-hydrogen enhance NMR sensitivity by polarization transfer. Science 323, 1708–1711 (2009). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources


