A scoping review of comparisons between abstracts and full reports in primary biomedical research
- PMID: 29287585
- PMCID: PMC5747940
- DOI: 10.1186/s12874-017-0459-5
A scoping review of comparisons between abstracts and full reports in primary biomedical research
Abstract
Background: Evidence shows that research abstracts are commonly inconsistent with their corresponding full reports, and may mislead readers. In this scoping review, which is part of our series on the state of reporting of primary biomedical research, we summarized the evidence from systematic reviews and surveys, to investigate the current state of inconsistent abstract reporting, and to evaluate factors associated with improved reporting by comparing abstracts and their full reports.
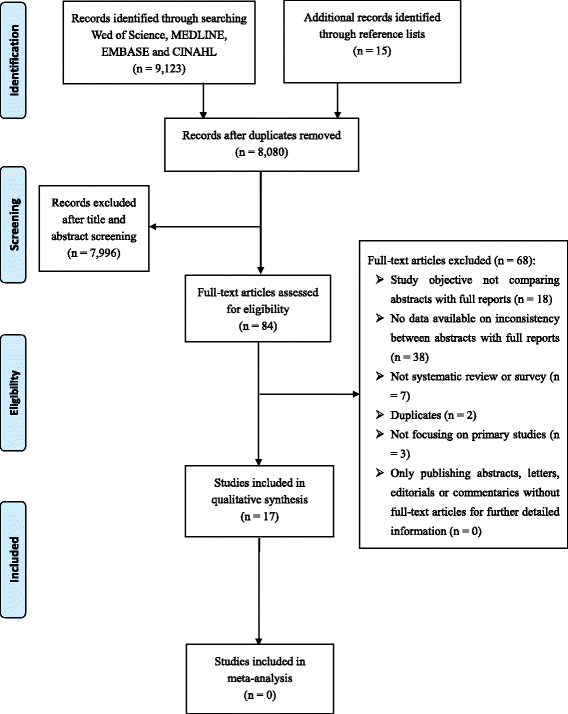
Methods: We searched EMBASE, Web of Science, MEDLINE, and CINAHL from January 1st 1996 to September 30th 2016 to retrieve eligible systematic reviews and surveys. Our primary outcome was the level of inconsistency between abstracts and corresponding full reports, which was expressed as a percentage (with a lower percentage indicating better reporting) or categorized rating (such as major/minor difference, high/medium/low inconsistency), as reported by the authors. We used medians and interquartile ranges to describe the level of inconsistency across studies. No quantitative syntheses were conducted. Data from the included systematic reviews or surveys was summarized qualitatively.
Results: Seventeen studies that addressed this topic were included. The level of inconsistency was reported to have a median of 39% (interquartile range: 14% - 54%), and to range from 4% to 78%. In some studies that separated major from minor inconsistency, the level of major inconsistency ranged from 5% to 45% (median: 19%, interquartile range: 7% - 31%), which included discrepancies in specifying the study design or sample size, designating a primary outcome measure, presenting main results, and drawing a conclusion. A longer time interval between conference abstracts and the publication of full reports was found to be the only factor which was marginally or significantly associated with increased likelihood of reporting inconsistencies.
Conclusions: This scoping review revealed that abstracts are frequently inconsistent with full reports, and efforts are needed to improve the consistency of abstract reporting in the primary biomedical community.
Keywords: Abstract; Accuracy; Deficiency; Discrepancy; Inconsistent reporting; Scoping review; Spin.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
A systematic review of comparisons between protocols or registrations and full reports in primary biomedical research.BMC Med Res Methodol. 2018 Jan 11;18(1):9. doi: 10.1186/s12874-017-0465-7. BMC Med Res Methodol. 2018. PMID: 29325533 Free PMC article.
-
State of reporting of primary biomedical research: a scoping review protocol.BMJ Open. 2017 Mar 29;7(3):e014749. doi: 10.1136/bmjopen-2016-014749. BMJ Open. 2017. PMID: 28360252 Free PMC article. Review.
-
Abstract analysis method facilitates filtering low-methodological quality and high-bias risk systematic reviews on psoriasis interventions.BMC Med Res Methodol. 2017 Dec 29;17(1):180. doi: 10.1186/s12874-017-0460-z. BMC Med Res Methodol. 2017. PMID: 29284417 Free PMC article.
-
Assessment of reporting quality of conference abstracts in sports injury prevention according to CONSORT and STROBE criteria and their subsequent publication rate as full papers.BMC Med Res Methodol. 2012 Apr 11;12:47. doi: 10.1186/1471-2288-12-47. BMC Med Res Methodol. 2012. PMID: 22494412 Free PMC article.
-
Assessment of the abstract reporting of systematic reviews of dose-response meta-analysis: a literature survey.BMC Med Res Methodol. 2019 Jul 15;19(1):148. doi: 10.1186/s12874-019-0798-5. BMC Med Res Methodol. 2019. PMID: 31307388 Free PMC article. Review.
Cited by
-
Disciplinary Imbalances in Urology and Gynecology Research Publications within Functional Urology.Clin Pract. 2024 Aug 29;14(5):1744-1752. doi: 10.3390/clinpract14050139. Clin Pract. 2024. PMID: 39311289 Free PMC article.
-
ENGINE-An EHS Project for Future Guidelines.J Abdom Wall Surg. 2024 Jul 12;3:13007. doi: 10.3389/jaws.2024.13007. eCollection 2024. J Abdom Wall Surg. 2024. PMID: 39071940 Free PMC article.
-
Adoption of an Enhanced Recovery After Surgery Protocol Increases Cost of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy and Does not Improve Outcomes.Ann Surg Oncol. 2024 Aug;31(8):5390-5399. doi: 10.1245/s10434-024-15320-x. Epub 2024 May 22. Ann Surg Oncol. 2024. PMID: 38777898
-
REporting quality of PilOt randomised controlled trials in surgery (REPORTS): a methodological survey protocol.BMJ Open. 2024 Apr 23;14(4):e085293. doi: 10.1136/bmjopen-2024-085293. BMJ Open. 2024. PMID: 38658008 Free PMC article.
-
Methotrexate treatment strategies for rheumatoid arthritis: a scoping review on doses and administration routes.BMC Rheumatol. 2024 Mar 5;8(1):11. doi: 10.1186/s41927-024-00381-y. BMC Rheumatol. 2024. PMID: 38444043 Free PMC article.
References
-
- Simera I, Altman DG. Writing a research article that is “fit for purpose”: EQUATOR network and reporting guidelines. Evidence Based. Medicine. 2009;14(5):132–134. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources