Odours of Plasmodium falciparum-infected participants influence mosquito-host interactions
- PMID: 28839251
- PMCID: PMC5570919
- DOI: 10.1038/s41598-017-08978-9
Odours of Plasmodium falciparum-infected participants influence mosquito-host interactions
Abstract
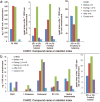
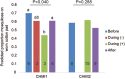
Malaria parasites are thought to influence mosquito attraction to human hosts, a phenomenon that may enhance parasite transmission. This is likely mediated by alterations in host odour because of its importance in mosquito host-searching behaviour. Here, we report that the human skin odour profile is affected by malaria infection. We compared the chemical composition and attractiveness to Anopheles coluzzii mosquitoes of skin odours from participants that were infected by Controlled Human Malaria Infection with Plasmodium falciparum. Skin odour composition differed between parasitologically negative and positive samples, with positive samples collected on average two days after parasites emerged from the liver into the blood, being associated with low densities of asexual parasites and the absence of gametocytes. We found a significant reduction in mosquito attraction to skin odour during infection for one experiment, but not in a second experiment, possibly due to differences in parasite strain. However, it does raise the possibility that infection can affect mosquito behaviour. Indeed, several volatile compounds were identified that can influence mosquito behaviour, including 2- and 3-methylbutanal, 3-hydroxy-2-butanone, and 6-methyl-5-hepten-2-one. To better understand the impact of our findings on Plasmodium transmission, controlled studies are needed in participants with gametocytes and higher parasite densities.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
No evidence for manipulation of Anopheles gambiae, An. coluzzii and An. arabiensis host preference by Plasmodium falciparum.Sci Rep. 2017 Aug 25;7(1):9415. doi: 10.1038/s41598-017-09821-x. Sci Rep. 2017. PMID: 28842622 Free PMC article.
-
Plasmodium falciparum gametocyte-induced volatiles enhance attraction of Anopheles mosquitoes in the field.Malar J. 2020 Sep 4;19(1):327. doi: 10.1186/s12936-020-03378-3. Malar J. 2020. PMID: 32887614 Free PMC article.
-
Mechanisms of Plasmodium-Enhanced Attraction of Mosquito Vectors.Trends Parasitol. 2017 Dec;33(12):961-973. doi: 10.1016/j.pt.2017.08.010. Epub 2017 Sep 21. Trends Parasitol. 2017. PMID: 28942108 Review.
-
Malaria infection increases attractiveness of humans to mosquitoes.PLoS Biol. 2005 Sep;3(9):e298. doi: 10.1371/journal.pbio.0030298. Epub 2005 Aug 9. PLoS Biol. 2005. PMID: 16076240 Free PMC article.
-
[Biology of man-mosquito Plasmodium transmission].Bull Soc Pathol Exot. 2003 Mar;96(1):6-20. Bull Soc Pathol Exot. 2003. PMID: 12784587 Review. French.
Cited by
-
Breath Biomarkers of Pediatric Malaria: Reproducibility and Response to Antimalarial Therapy.J Infect Dis. 2024 Oct 16;230(4):1013-1022. doi: 10.1093/infdis/jiae323. J Infect Dis. 2024. PMID: 38885291
-
À la carte: how mosquitoes choose their blood meals.Trends Parasitol. 2024 Jul;40(7):591-603. doi: 10.1016/j.pt.2024.05.007. Epub 2024 Jun 9. Trends Parasitol. 2024. PMID: 38853076 Review.
-
Smelly interactions: host-borne volatile organic compounds triggering behavioural responses in mosquitoes, sand flies, and ticks.Parasit Vectors. 2024 May 16;17(1):227. doi: 10.1186/s13071-024-06299-1. Parasit Vectors. 2024. PMID: 38755646 Free PMC article. Review.
-
Metabolites From Trypanosome-Infected Cattle as Sensitive Biomarkers for Animal Trypanosomosis.Front Microbiol. 2022 Jul 14;13:922760. doi: 10.3389/fmicb.2022.922760. eCollection 2022. Front Microbiol. 2022. PMID: 35910617 Free PMC article.
-
Experiment in semi-natural conditions did not confirm the influence of malaria infection on bird attractiveness to mosquitoes.Parasit Vectors. 2022 Jun 2;15(1):187. doi: 10.1186/s13071-022-05292-w. Parasit Vectors. 2022. PMID: 35655262 Free PMC article.
References
-
- Hughes, D. P., Brodeur, J. & Thomas, F. Host Manipulation By Parasites. (Oxford University Press, 2012).
-
- Dawkins, R. The Extended Phenotype. (Oxford University Press, 1982).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical


