30 YEARS OF THE MINERALOCORTICOID RECEPTOR: Mineralocorticoid receptor antagonists: 60 years of research and development
- PMID: 28634268
- PMCID: PMC5488394
- DOI: 10.1530/JOE-16-0600
30 YEARS OF THE MINERALOCORTICOID RECEPTOR: Mineralocorticoid receptor antagonists: 60 years of research and development
Abstract
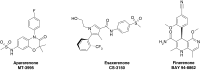
The cDNA of the mineralocorticoid receptor (MR) was cloned 30 years ago, in 1987. At that time, spirolactone, the first generation of synthetic steroid-based MR antagonists (MRAs), which was identified in preclinical in vivo models, had already been in clinical use for 30 years. Subsequent decades of research and development by Searle & Co., Ciba-Geigy, Roussel Uclaf and Schering AG toward identifying a second generation of much more specific steroidal MRAs were all based on the initial 17-spirolactone construct. The salient example is eplerenone, first described in 1987, coincidentally with the cloning of MR cDNA. Its launch on the market in 2003 paralleled intensive drug discovery programs for a new generation of non-steroidal MRAs. Now, 30 years after the cDNA cloning of MR and 60 years of clinical use of steroidal MRAs, novel non-steroidal MRAs such as apararenone, esaxerenone and finerenone are in late-stage clinical trials in patients with heart failure, chronic kidney disease (CKD), hypertension and liver disease. Finerenone has already been studied in over 2000 patients with heart failure plus chronic kidney disease and/or diabetes, and in patients with diabetic kidney disease, in five phase II clinical trials. Here, we reflect on the history of the various generations of MRAs and review characteristics of the most important steroidal and non-steroidal MRAs.
Keywords: apararenone; canrenone; eplerenone; esaxerenone; finerenone; mineralocorticoid receptor antagonists; potassium canrenoate; spironolactone.
© 2017 The authors.
Figures


Similar articles
-
Effectiveness of nonsteroidal mineralocorticoid receptor antagonists in patients with diabetic kidney disease.Postgrad Med. 2023 Apr;135(3):224-233. doi: 10.1080/00325481.2022.2060598. Epub 2022 Apr 20. Postgrad Med. 2023. PMID: 35392754 Review.
-
Steroidal and Novel Non-steroidal Mineralocorticoid Receptor Antagonists in Heart Failure and Cardiorenal Diseases: Comparison at Bench and Bedside.Handb Exp Pharmacol. 2017;243:271-305. doi: 10.1007/164_2016_76. Handb Exp Pharmacol. 2017. PMID: 27830348 Review.
-
Non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease.Eur Heart J. 2022 Aug 14;43(31):2931-2945. doi: 10.1093/eurheartj/ehac299. Eur Heart J. 2022. PMID: 35713973
-
Mineralocorticoid Receptor Blockers: Novel Selective Nonsteroidal Mineralocorticoid Receptor Antagonists.Curr Hypertens Rep. 2020 Feb 29;22(3):21. doi: 10.1007/s11906-020-1023-y. Curr Hypertens Rep. 2020. PMID: 32114686 Review.
-
Cardiovascular and Renal Benefit of Novel Non-steroidal Mineralocorticoid Antagonists in Patients with Diabetes.Curr Cardiol Rep. 2023 Dec;25(12):1859-1864. doi: 10.1007/s11886-023-01998-0. Epub 2023 Nov 22. Curr Cardiol Rep. 2023. PMID: 37991625 Review.
Cited by
-
Possible mechanisms of SARS-CoV-2-associated myocardial fibrosis: reflections in the post-pandemic era.Front Microbiol. 2024 Oct 8;15:1470953. doi: 10.3389/fmicb.2024.1470953. eCollection 2024. Front Microbiol. 2024. PMID: 39444690 Free PMC article. Review.
-
Mineralocorticoid receptor antagonists and heart failure with preserved ejection fraction: current understanding and future prospects.Heart Fail Rev. 2024 Oct 17. doi: 10.1007/s10741-024-10455-1. Online ahead of print. Heart Fail Rev. 2024. PMID: 39414721 Review.
-
A comprehensive review of finerenone-a third-generation non-steroidal mineralocorticoid receptor antagonist.Front Cardiovasc Med. 2024 Sep 23;11:1476029. doi: 10.3389/fcvm.2024.1476029. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39376623 Free PMC article. Review.
-
Finerenone in heart failure and chronic kidney disease with type 2 diabetes: FINE-HEART pooled analysis of cardiovascular, kidney and mortality outcomes.Nat Med. 2024 Dec;30(12):3758-3764. doi: 10.1038/s41591-024-03264-4. Epub 2024 Sep 1. Nat Med. 2024. PMID: 39218030
-
Clinical Properties and Non-Clinical Testing of Mineralocorticoid Receptor Antagonists in In Vitro Cell Models.Int J Mol Sci. 2024 Aug 22;25(16):9088. doi: 10.3390/ijms25169088. Int J Mol Sci. 2024. PMID: 39201774 Free PMC article. Review.
References
-
- Aldactone NDA. 2008. Aldactone, spironolactone tablets USP, NDA 12-151/S-062, Pfizer, distributed by G.D. Searle LLC, LAB-0231-4.0, revised January 2008. New York, NY, USA: Pfizer Inc. (available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/012151s062lbl.pdf)
-
- Amazit L, Le Billan F, Kolkhof P, Lamribet K, Viengchareun S, Fay MR, Khan JA, Hillisch A, Lombès M, Rafestin-Oblin ME, et al. 2015. Finerenone impedes aldosterone-dependent nuclear import of the mineralocorticoid receptor and prevents genomic recruitment of steroid receptor coactivator-1. Journal of Biological Chemistry 290 21876–21889. (10.1074/jbc.M115.657957) - DOI - PMC - PubMed
-
- Arai K, Homma T, Morikawa Y, Ubukata N, Tsuruoka H, Aoki K, Ishikawa H, Mizuno M, Sada T. 2015a. Pharmacological profile of CS-3150, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist. European Journal of Pharmacology 761 226–234. (10.1016/j.ejphar.2015.06.015) - DOI - PubMed
-
- Arai K, Morikawa Y, Ubukata N, Tsuruoka H, Homma T. 2016. CS-3150, a novel nonsteroidal mineralocorticoid receptor antagonist, shows preventive and therapeutic effects on renal injury in deoxycorticosterone acetate/salt-induced hypertensive rats. Journal of Pharmacology and Experimental Therapeutics 358 548–557. (10.1124/jpet.116.234765) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials


